Hierarchical alginate biopolymer papers produced via lanthanide ion coordination†
Abstract
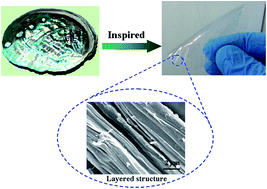
Inspired by the heterogeneous architectures of biological composites, mimicking the hierarchical structure of nacre is a powerful strategy to construct high-performance materials. This paper presents a lightweight and nacre-like hierarchical paper which was fabricated via lanthanide ion coordination. Sodium alginate (SA) biopolymers and lanthanide ions (Nd3+, Gd3+, Ce3+ and Yb3+) were used as ideal building blocks and connection points, respectively. SA biopolymers and lanthanide ions rapidly self-assembled into an aligned hydrogel. The synthesized hydrogel was subsequently dried to form layered alginate-based papers. The formation mechanism of the layered paper was investigated and demonstrated that lanthanide ion coordination can produce the hierarchical structure. The as-prepared layered SA–Nd(III) nanopaper exhibited a high strength of 124.2 ± 5.2 MPa, toughness of 8.2 ± 0.4 MJ m−3, and Young's modulus of 5.2 ± 0.2 GPa, as well as excellent resistance to solvents. Owing to their outstanding mechanical properties and easy and fast fabrication, the layered SA–Nd(III) papers demonstrated a potential application in the fields of biomaterials. This new strategy based on lanthanide ion coordination, can also be used to construct integrated, high-performance, and biopolymer materials.

 Please wait while we load your content...
Please wait while we load your content...