Supersaturation-dependent polymorphic outcome and transformation rate of L-glutamic acid†
Shanshan Liang,
Xuezhi Duan,
Xiangyang Zhang,
Gang Qian* and
Xinggui Zhou
State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai, 200237, China. E-mail: carlqg@ecust.edu.cn
First published on 2nd August 2016
Abstract
A link between the predominant conformer in supersaturated L-glutamic acid aqueous solution detected by FTIR spectroscopy and the polymorphic outcome is reported. In particular, the supersaturation is found to determine the morphology of the initially crystallized α form and thus to affect the rate for its further transformation to the β form.
The control of polymorphs has become an important issue in both science and industry, since different polymorphs of the same species usually exhibit different physical and chemical properties.1,2 However, achieving control of a specific polymorph remains a significant challenge because of the limited fundamental understanding of the factors dictating the polymorphic outcome.
Traditionally, polymorphic formation is known to be affected by many conditions, such as supersaturation, temperature, solvent, and additives.3,4 In particular, supersaturation, the driving force for the crystallization process, is of utmost significance, which places the crystallization process under either kinetic or thermodynamic control.5,6 The mechanism of supersaturation dependent nucleation control, however, is still as an open question.
More and more investigations have revealed that solute molecules tend to self-assemble into different structural motifs in supersaturated solution.7–9 In some cases, the structure of most abundant pre-nucleation aggregates is directly corresponded to the structure in the polymorph initially crystallized. For example, the 2,6-dihydroxybenzoic acid molecules have been found to associate into dimers in toluene and catemers in chloroform via UV spectroscopy characterization. As a result, crystallization from toluene leads to form 1 with the dimers crystal structure and from chloroform leads to form 2 with the catemers crystal structure.10 However, some reports concluded that the structure of clusters in supersaturated solution had little resemblance to that of the resulting crystal in recent years.11 These efforts illustrate that the nature of molecular self-assembly leading to nucleation is complicated and has not been completely understood.
L-Glutamic acid (L-Glu) is a relatively well studied model compound with considerable industrial relevance. It has two known polymorphs, i.e., the metastable α form and the stable β form.12,13 The crystal chemistry of the two polymorphs shown in Fig. S1,† is characterized by hydrogen-bonded ring structures in the case of the α form and hydrogen-bonded zigzag chains in the case of the β form. The molecular conformations present in the two polymorphs (see Fig. S2†) are quite different, especially with respect to their side chain, with the α form molecule adopting a more twisted conformation.3,14 In addition, the granular α form in comparison with the needle-like β form is preferred industrially for its good separability from the mother liquor.15,16 Unfortunately, the α form is prone to transform to the β form via solution-mediated polymorphic transformation (SMPT), in which the β form can nucleate on the surfaces of the α form.17–22 In recent years, the supersaturation-dependent polymorphic nucleation control of L-Glu has been achieved much attention, as the process is comparatively simple and would not incorporate any nucleation selecting additives into the mother solution.23–25 However, the origin of supersaturation-dependent polymorphic nucleation control is not well elucidated. In the present work, the FTIR spectroscopy, which has been proved as one of the most useful tools for identification of the structural motifs in solution and the final solid form,9,26,27 was implemented to study solution chemistry in order to establish a relationship between the species self-association in supersaturated solution and the polymorphic outcome. Furthermore, the supersaturation effect on transformation rate from α to β form was also studied. The insights revealed herein may provide some fundamental understanding of the polymorphic crystallization.
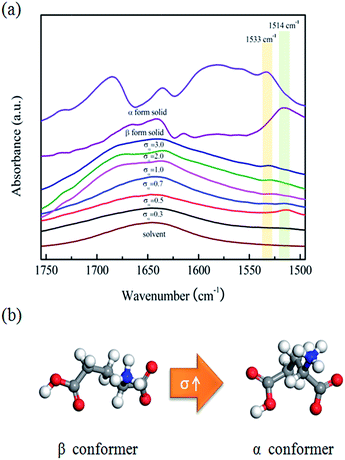
First, FTIR spectroscopy was used to monitor the crystallization of L-Glu from different supersaturated solutions at 25 °C, and to characterize the solid α and β form. As shown in Fig. S3,† the solid state spectra of the two polymorphs of L-Glu are clearly different, and thus are suitable for such experiments. However, because of the low solubility of L-Glu in water and the interference of the water, the most characteristic peaks for α and β form in solution are overlapped by those of water. Fortunately, the peaks at 1533 or 1514 cm−1 respectively corresponding to the bending vibration of N–H for the α and β form can be distinguished in the solution spectra of different supersaturated L-Glu solutions, as depicted in Fig. 1(a). Clearly, with the increase of supersaturation, the position of the N–H peak moves from 1514 to 1533 cm−1. This suggests the different hydrogen bonding networks in different supersaturated solutions, which may induce a change in molecular conformation, and thus lead to the predominant conformer in solution transforming from the β to the α conformer. In detail, at lower supersaturation (σα ≤ 0.5), peak at 1514 cm−1 is observed, indicating the β conformer being the predominant one in the solution, while at higher supersaturation (σα ≥ 1.0), the peak shifts to 1533 cm−1, suggesting that the α conformer has become the predominant one. Meanwhile, at an intermediate supersaturation (e.g., σα = 0.7), both the peaks at 1514 and 1533 cm−1 can be seen, which means that the two conformers may exist simultaneously in the solution. These results imply that with the increase of the supersaturation, the predominant conformer in solution transforms from the β to the α conformer, as presented in Fig. 1(b).
It can be assumed that the major conformers in supersaturated solution may form clusters, and thus the corresponding polymorphous crystals may precipitate.3 Moreover, since the two polymorphs of L-Glu have their typical habits, the initial form to crystallize could be identified from their characteristic crystal habits. Therefore, the nucleation processes were monitored by in situ optical microscopy.
The typical microscopic images of the crystals nucleated from different supersaturated solutions are displayed in Fig. S4.† At lower supersaturation level (σα ≤ 0.5), only needle-like crystals (β form) can be observed (see Fig. S4(a) and (b)†). In the intermediate supersaturation (e.g., σα = 0.7), as shown in Fig. S4(c),† both the granular crystals (α form) and the needle-like crystals (β form) can be seen. Further increase in the supersaturation (σα ≥ 1.0) favours only the nucleation of granular crystals (Fig. S4(d)†).
Off line PXRD was further employed to determine polymorphic compositions of the nucleated crystals.28 In detail, the characteristic peaks at 18.2° and 26.6° (2θ) for the α form, and 10.2°, 21.4° and 25.6° (2θ) for the β form are selected for identification and quantification of L-Glu. The obtained PXRD patterns for the different mixture samples are shown in Fig. S5(a).† Linear regression analysis between mass fraction of the α form in mixture and peak intensity ratio of the α form in the PXRD profiles is calculated, and the calibration line (Fig. S5(b)†) displays a good agreement with the data. Hence, the ratio of the α form in the mixture is analyzed using this method.
The PXRD patterns shown in Fig. S6† validate that only the stable β form nucleated from the solution with supersaturation range lower than 0.5 as only the characteristic peaks of β form exist.12 At the supersaturation of σα = 0.7, both the characteristic peaks of α and β form can be identified (see Fig. S7†). Further quantitative analysis shows that the nucleated crystal is a mixture of the two polymorphs with 64 wt% α form. In the higher supersaturation range (σα ≥1.0), the characteristic peaks of β form disappear and only those of α form can be identified, suggesting that only the nucleation of α form occurs.
Both the morphologies and PXRD patterns of the nucleated crystals reveal that only β or α form can nucleate respectively in the lower or higher supersaturation range, while the two polymorphs can nucleate concomitantly at an intermediate supersaturation range, as summarized in Table 1, which is similar as the findings of Srinivasan et al. using swift cooling method.23,24 These results coincide well with those of the FTIR spectroscopy as above obtained, indicating a clear connection between the predominant conformers in solution and the final polymorphic outcome.
| supersaturation (σα) | crystal form |
|---|---|
| 0.3 | β |
| 0.5 | β |
| 0.7 | α + β |
| 1.0 | α |
| 2.0 | α |
| 3.0 | α |
Moreover, it has been found that in the higher supersaturation range (σα ≥ 1.0), although the nucleated crystals are all the α form, their morphologies are supersaturation-dependent.29,30 As shown in Fig. 2, with the increase of the supersaturation, both the ratios of the {011} and {111} surfaces of α form increase, while that of {001} surface decreases. As has been reported, the first nucleated α form will transform to β form through SMPT, in which β form can nucleate on the surfaces of α form with the probability following the order of {011} > {111} > {001}.31,32 Therefore, it is reasonable to deduce that the morphology change of α form with supersaturation will result in supersaturation-dependent transformation rate of L-Glu.
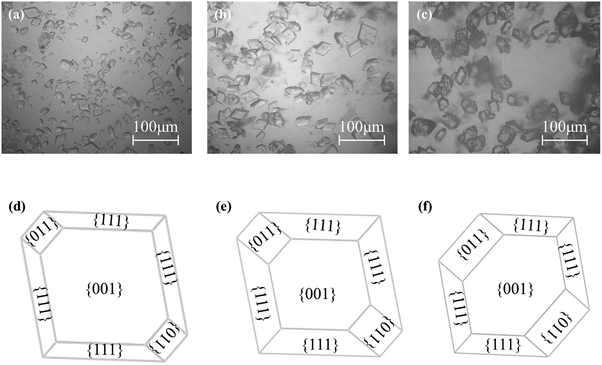 | ||
| Fig. 2 Optical micrographs and schematic morphologies of the three different nucleated polymorphs. (a) and (d) at σα = 1.0, (b) and (e) at σα = 2.0, and (c) and (f) at σα = 3.0. | ||
In order to determine the transformation time, PXRD was further employed to quantitatively analyse the polymorphic composition of the crystals taken at different intervals during the SMPT of L-Glu. A typical PXRD result with the supersaturation of σα = 1.0 is presented in Fig. 3. At the beginning, only the characteristic peaks of α form are identified, i.e., all the crystals are α form (see Fig. 3(a)). With the time elapsed, the percentage of the β form increases at the expense of α form consumed as reflected in the change of relative strength of the characteristic peaks for the two polymorphs. For example, at 12 h, the mass weight of β form increases to 48% (see Fig. 3(b)). Finally, at 24 h, only the characteristic peaks of β form are observed (see Fig. 3(c)), indicating the complete transformation of α to β form. Furthermore, the transformation time of three highly supersaturated solutions of L-Glu is summarized in Fig. 4(a), and typical images for the SMPT of supersaturated L-Glu solution with σα = 3.0 are displayed in Fig. 4(b)–(d). As expected, with the supersaturation increasing from σα = 1.0 to 3.0, the transformation time is shortened quickly from 24 h to 1 h. These results confirm the above deduction and demonstrate that the change of α form morphology with supersaturation indeed affects the polymorphic transformation rate of L-Glu.
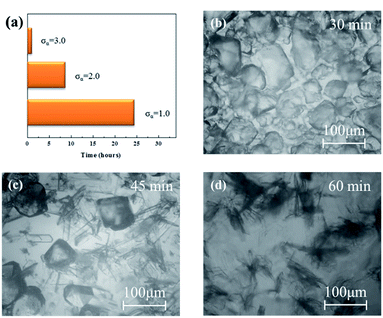 | ||
| Fig. 4 (a) The transformation time for different highly supersaturated solutions and (b–d) typical images for the SMPT of supersaturated L-Glu solution with σα = 3.0. | ||
In conclusion, the link between polymorphic outcomes of different supersaturated L-Glu solutions and the most abundant pre-nucleation aggregates in solution detected by FTIR spectroscopy has been revealed. Moreover, different highly supersaturated solutions result in the initially nucleated crystals of α form with different morphologies which causes the change in its subsequent transformation rate to β form.
Acknowledgements
This work is financially supported by the 111 Project of the Ministry of Education of China (B08021) and National Natural Science Foundation of China (NSFC, No. 21406071) and National Higher Education Institution General Research and Development Funding (No. 22A201514006).Notes and references
- A. Maher, D. M. Croker, Å. C. Rasmuson and B. K. Hodnett, Cryst. Growth Des., 2012, 12, 6151–6157 CAS.
- A. Llinàs and J. M. Goodman, Drug Discovery Today, 2008, 13, 198–210 CrossRef PubMed.
- M. Kitamura, J. Cryst. Growth, 2002, 237–239(3), 2205–2214 CrossRef CAS.
- M. Kitamura, CrystEngComm, 2009, 11, 949–964 RSC.
- N. C. S. Kee, R. B. H. Tan and R. D. Braatz, Cryst. Growth Des., 2009, 9, 3044–3051 CAS.
- C. Sudha and K. Srinivasan, CrystEngComm, 2013, 15, 1914–1921 RSC.
- C. S. Towler and L. S. Taylor, Cryst. Growth Des., 2007, 7, 633–638 CAS.
- A. Mattei and T. Li, Pharm. Res., 2012, 29, 460–470 CrossRef CAS PubMed.
- S. Parveen, R. J. Davey, G. Dent and R. G. Pritchard, Chem. Commun., 2005, 1531–1533 RSC.
- R. J. Davey, N. Blagden, S. Righini, H. Alison, M. J. Quayle and S. Fuller, Cryst. Growth Des., 2000, 1, 59–65 CrossRef.
- W. Du, A. J. Cruz-Cabeza, S. Woutersen, R. J. Davey and Q. Yin, Chem. Sci., 2015, 6, 3515–3524 RSC.
- J. Schöll, D. Bonalumi, L. Vicum, M. Mazzotti and M. Müller, Cryst. Growth Des., 2006, 6, 881–891 Search PubMed.
- Y. Mo, L. Dang and H. Wei, Ind. Eng. Chem. Res., 2011, 50, 10385–10392 CrossRef CAS.
- R. J. Davey, N. Blagden, G. D. Potts and R. Docherty, J. Am. Chem. Soc., 1997, 119, 1767–1772 CrossRef CAS.
- M. Kitamura, J. Cryst. Growth, 1989, 96, 541–546 CrossRef CAS.
- G. Qian, Y. Wu, X. Yang, X. Duan and X. Zhou, J. Cryst. Growth, 2013, 373, 78–81 CrossRef CAS.
- W. Ostwald, Z. Physiol. Chem., 1897, 22, 289–330 CAS.
- C. Cashell, D. Corcoran and B. K. Hodnett, Chem. Commun., 2003, 374–375 RSC.
- E. S. Ferrari and R. J. Davey, Cryst. Growth Des., 2004, 4, 1061–1068 CAS.
- D. Croker and B. K. Hodnett, Cryst. Growth Des., 2010, 10, 2806–2816 CAS.
- R. B. Hammond, K. Pencheva and K. J. Roberts, J. Phys. Chem. B, 2005, 109, 19550–19552 CrossRef CAS PubMed.
- R. B. Hammond, K. Pencheva and K. J. Roberts, Cryst. Growth Des., 2007, 7, 875–884 CAS.
- K. Srinivasan and P. Dhanasekaran, Amino Acids, 2011, 40, 1257–1260 CrossRef CAS PubMed.
- K. Srinivasan and P. Dhanasekaran, J. Cryst. Growth, 2011, 318, 1080–1084 CrossRef CAS.
- S. Khan, C. Y. Ma, T. Mahmud, R. Y. Penchev, K. J. Roberts, J. Morris, L. Özkan, G. White, B. Grieve, A. Hall, P. Buser, N. Gibson, P. Keller, P. Shuttleworth and C. J. Price, Org. Process Res. Dev., 2011, 15, 540–555 CrossRef CAS.
- R. Bobrovs, L. Seton and N. Dempster, CrystEngComm, 2015, 17, 5237–5251 RSC.
- A. Borissova, S. Khan, T. Mahmud, K. J. Roberts, J. Andrews, P. Dallin, Z.-P. Chen and J. Morris, Cryst. Growth Des., 2009, 9, 692–706 CAS.
- S. Dharmayat, R. B. Hammond, X. Lai, C. Ma, E. Purba, K. J. Roberts, Z.-P. Chen, E. Martin, J. Morris and R. Bytheway, Cryst. Growth Des., 2008, 8, 2205–2216 CAS.
- W. Tan, X. Yang, X. Duan, X. Zhang, G. Qian and X. Zhou, Cryst. Res. Technol., 2016, 51, 23–29 CrossRef CAS.
- H.-M. Shim, H.-S. Kim and K.-K. Koo, Cryst. Growth Des., 2015, 15, 1833–1842 CAS.
- S. Liang, X. Duan, X. Zhang, G. Qian and X. Zhou, Cryst. Growth Des., 2015, 15, 3602–3608 CAS.
- S. Liang, X. Duan, X. Zhang, G. Qian and X. Zhou, Chem. Eng. Technol., 2016, 39, 1295–1300 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, FTIR spectra of solid and solvent, PXRD profiles of L-Glu crystals with various compositions, microscopy images of nucleation observed at various supersaturation and PXRD profiles of crystals obtained from supersaturated L-Glu solution with σα = 0.3, 0.5 and 0.7, respectively. See DOI: 10.1039/c6ra11586a |
| This journal is © The Royal Society of Chemistry 2016 |