Regularities of deep deoxidization of molten ionic chlorides in reactive gas atmosphere
V. L. Cherginets*a,
T. P. Rebrovaa,
V. A. Naumenkoa,
A. L. Rebrova and
O. I. Yurchenkob
aInstitute for Scintillation Materials of National Academy of Sciences of Ukraine, Nauki Avenue, 60, Kharkov, 61001, Ukraine. E-mail: v_cherginets@ukr.net; Fax: +38 057 3404474; Tel: +38 057 3410218
bV. N. Karazin Kharkiv National University, Svobody Sq. 4, Kharkov, 61022, Ukraine
First published on 13th June 2016
Abstract
The process of the removal of oxide ion admixtures by the action of CCl4 vapor (carbochlorination) from chloride melts of KCl–NaCl (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), BaCl2–KCl (0.26
0.5), BaCl2–KCl (0.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.74) and KCl–LiCl (0.41
0.74) and KCl–LiCl (0.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.59) in the temperature range of 943–1023 K was studied by a potentiometric method using a Pt(O2)|YSZ oxygen electrode for the detection of the current concentration of oxide ions. The chemical stage of this process in all the molten mixtures was characterized by 2nd order kinetics with respect to oxide ions. The thermal dependence of the conditional rate constants gives the possibility of estimating the activation energies of the carbohalogenation as 233 ± 33 kJ mol−1, 238 ± 30 kJ mol−1 and 177 ± 30 kJ mol−1, respectively. The purification limits (residual concentration of O2−) of the said melts in the 943–1023 K temperature range are (1 ÷ 5) × 10−9 mol kg−1 for KCl–NaCl, (1 ÷ 2) × 10−7 mol kg−1 for BaCl2–KCl and (1 ÷ 2) × 10−6 mol kg−1 for KCl–LiCl. The shift of position of the said purification limit with the change of cation composition of the melt agrees with the changes of the oxoacidic properties (the oxobasicity indices) of the melt.
0.59) in the temperature range of 943–1023 K was studied by a potentiometric method using a Pt(O2)|YSZ oxygen electrode for the detection of the current concentration of oxide ions. The chemical stage of this process in all the molten mixtures was characterized by 2nd order kinetics with respect to oxide ions. The thermal dependence of the conditional rate constants gives the possibility of estimating the activation energies of the carbohalogenation as 233 ± 33 kJ mol−1, 238 ± 30 kJ mol−1 and 177 ± 30 kJ mol−1, respectively. The purification limits (residual concentration of O2−) of the said melts in the 943–1023 K temperature range are (1 ÷ 5) × 10−9 mol kg−1 for KCl–NaCl, (1 ÷ 2) × 10−7 mol kg−1 for BaCl2–KCl and (1 ÷ 2) × 10−6 mol kg−1 for KCl–LiCl. The shift of position of the said purification limit with the change of cation composition of the melt agrees with the changes of the oxoacidic properties (the oxobasicity indices) of the melt.
Introduction
Chloride ionic melts are now widely used for many industrial and scientific purposes. They serve as media for obtaining the most active metals, and as solvents for high-temperature chemical power sources etc.1–3 One of the most developing modern application of mixed melts based on alkali metal chlorides is their usage as initial media for the growth of novel halide optical crystals.4,5 For a majority of these purposes, the presence of oxygen-containing admixtures in the melts seems very undesirable because of the worsening of the quality of the final products and their functional characteristics. All the stages in the preparation of chloride melts are accompanied by the entering of oxygen-containing admixtures into the raw or resulting melt due to the interactions of water vapor, carbon dioxide and oxygen with solid or liquid chlorides, resulting in their pyrohydrolysis and oxidation. Therefore, deoxidization of the obtained melts presents an important problem for scientists working in the above-mentioned fields. One of the simplest ways of deoxidization is the treatment of the chloride melts by chloro-derivatives of hydrocarbons with a high content of chlorine, and tetrachloromethane CCl4 is the most convenient compound for this purpose since it is inert enough at room temperature, slightly toxic and possesses a relatively high saturated vapor pressure.The process of carbochlorination using CCl4 can be described by the following simple equation:
| CCl4↑ + 2O2− → CO2↑ + 4Cl−. | (1) |
Tetrachloromethane can be considered as the main chlorinating agent at temperatures of the order 1000–1100 K, although thermodynamic calculations predict the complete transformation of CCl4 in the decomposition products (C2Cl4, C, Cl2 etc.). Nevertheless, authors6 have studied process of thermal decomposition of CCl4 in molten chlorides and showed that the degree of conversion of tetrachloromethane into the mentioned products did not exceed 0.4, even after passing CCl4 through a layer of chloride melt of 130 mm height.
Although the successful use of carbochlorination by CCl4 for the purification of several chloride melts was reported,7 some essential features of the purification process, such as the rate of carbochlorination and the effect of temperature on it, the purification limit, and the effect of the melt composition (and oxoacidity) remain unclear. The consideration of these questions is the main goal of the presented paper where the process of carbochlorination is studied in the KCl–NaCl (equimolar) → BaCl2–KCl (eutectic, 0.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.74) → KCl–LiCl (eutectic, 0.41
0.74) → KCl–LiCl (eutectic, 0.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.59) sequence in a wide temperature range. The choice of the melt sequence is due to the fact that their acidic properties vary in wide range: the oxobasicity indices pIL (concentration analog of the primary medium effect for O2− for the melt L against a KCl–NaCl equimolar mixture) increase as follows: 0 → 1.83 → 3.5.8 The existence of a clear correlation between the melt oxoacidity and any of the purification characteristics will give the possibility to predict (by interpolation or juxtaposition) similar parameters for SrCl2-based chloride melts (pIL is ca. 2 (ref. 8)) or those based on CaCl2 (pIL is ca. 4 (ref. 8)).
0.59) sequence in a wide temperature range. The choice of the melt sequence is due to the fact that their acidic properties vary in wide range: the oxobasicity indices pIL (concentration analog of the primary medium effect for O2− for the melt L against a KCl–NaCl equimolar mixture) increase as follows: 0 → 1.83 → 3.5.8 The existence of a clear correlation between the melt oxoacidity and any of the purification characteristics will give the possibility to predict (by interpolation or juxtaposition) similar parameters for SrCl2-based chloride melts (pIL is ca. 2 (ref. 8)) or those based on CaCl2 (pIL is ca. 4 (ref. 8)).
Additionally to the systematic study of the carbohalogenation of molten bromides5 the proposed paper contains some generalizations of these recent results.
Experimental
The molten chloride mixtures for the investigations were obtained from BaCl2, KCl, LiCl (glass ampoules with sealed end) and NaCl of reagent quality (‘Reakhim’, Russia). Excluding LiCl, the salts were dried in air at 423–473 K for 2 h. The raw compositions (mass fractions) were 0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.44 for the KCl–NaCl equimolar mixture, 0.50
0.44 for the KCl–NaCl equimolar mixture, 0.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 for the BaCl2–KCl eutectic and 0.55
0.50 for the BaCl2–KCl eutectic and 0.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45 for the KCl–LiCl eutectic.
0.45 for the KCl–LiCl eutectic.
Used as standard oxide ion donor, KOH (99.9%) was melted in an alundum crucible and was kept for 1 h at 700 °C in an argon atmosphere that provided the complete removal of absorbed water. Such a treatment did not cause the appreciable contamination of smelted KOH by aluminum and its content was not more than 0.34 mass% (AES ICP analysis), so could not distort the obtained results.
To create an inert atmosphere in the potentiometric cell, we used high-purity Ar (the volume fraction of the main substance was 0.9999), preliminarily dried by passing over P2O5 that provided deep purification from traces of H2O. This gas was used as a gas-carrier for performing the carbohalogenation. The volume fraction of free oxygen in the dried gas was not more than 2 × 10−5.
The scheme of the potentiometric cell for the determination of equilibrium O2− molality is as follows:
| Ag|Ag+ + melt‖melt + O2−|YSZ|Pt(O2), | (2) |
| 2KOH → 2K+ + H2O↑ + O2− | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mO2−. The molalities of oxide ion during the carbochlorination process were calculated using the calibration data which were different for each melt and temperature.
mO2−. The molalities of oxide ion during the carbochlorination process were calculated using the calibration data which were different for each melt and temperature.
The carbohalogenation routine consisted of the following: after calibration, the oxide ion concentration in the studied melt was reduced to pO values within 3–4 by the addition of ammonium chloride, the action of which is expressed as follows:
| 2NH4Cl + O2− → 2NH3↑ + H2O↑ + 2Cl−. | (4) |
To study the kinetic parameters of the carbochlorination process, a flow of argon saturated by CCl4 vapor at 293 K was passed through the solution at a rate of 150 mL min−1, which should provide stable concentrations of CCl4 and the products of its pyrolysis in the reactive gas atmosphere over the melt. pO changes were fixed each 30 s during the first 5 min of the bubbling of the gas mixture, then the intervals between the consecutive measurements was increased to 1, 2 and, finally, 5 min. The experiments were performed at 943 (953 K for the KCl–NaCl mixture), 973, 1000 and 1023 K. It should be noted that according to the data of handbooks11,12 the melting points are 933 K for KCl–NaCl, 916 K for BaCl2–KCl and 625 K for KCl–LiCl eutectic mixtures. Lower experimental temperatures were chosen to provide some overheating which helped to avoid experimental difficulties associated with some non-uniformity of the thermal profile of the furnace.
Results and discussion
The effect of melt compositions and temperature on the deoxidization process
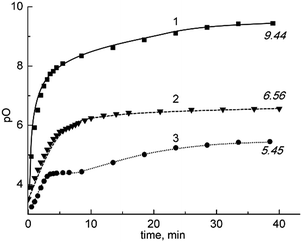
Examples of the typical shapes of dependences constructed on the basis of the experimental data for all investigated melts at 1000 K are depicted in Fig. 1. As is seen, the treatment process in all cases is characterized by the monotonous increase of pO with time up to a plateau where the purification process stops. The degree of purification decreases in the following sequence KCl–NaCl → BaCl2–KCl → KCl–LiCl, which can be explained by the strengthening of the oxoacidic properties of the melts in the said sequence.Another distinction of the carbochlorination consists of the fact that although the curve obtained for molten KCl–NaCl, which possesses the weakest oxoacidic properties, can be considered as steady and smooth, for other melts with cations possessing a higher affinity to oxide ions (Ba2+ and, especially, Li+) there is some slowdown of the pO growth in the 5–10 min time range.
This also can be explained by the formation of BaO and Li2O in the corresponding chloride melts and their destruction by the action of the chlorinating agent requires additional time. A similar phenomenon was observed by Castrillejo et al.13,14 who passed gaseous hydrogen chloride through melts of KCl–LiCl and CaCl2–NaCl compositions containing suspended rare earth oxochlorides. The passing of HCl through the melt resulted in the process:
| LnOCl + 2HCl → H2O↓ + LnCl3. | (5) |
The dependence of pO vs. time also included inflections caused by the slow stage (5).
Finally, the end of the treatment process corresponds to the plateau section. The plateau is explained by features usual for purification processes: practically every such process is accompanied by contamination of the purified substance because of its contact with the atmosphere and construction materials. For the experiments we used an alundum (Al2O3) crucible, electrodes in alumina and zirconia test-tubes immersed into the melt and argon containing traces of O2 and a very small amount of water. The following processes:
| Al2O3↓ → 2AlO+ + O2−, | (6) |
| O2↑ + 4Cl− → 2O2− + 2Cl2↑, | (7) |
| H2O↑ + 2Cl− → O2− + 2HCl↑, | (8) |
Returning to the effect of temperature on the efficiency of the purification process, it can be noted that the increase of the temperature, as a rule, makes the purification deeper.
So, the rise of temperature (80 K) results in an increase of pO by approximately 1 (the changes in pO are from 8.6 to 9.5, Fig. 2).15 The corresponding changes for other melts are from 6.6 to 7.1 for the BaCl2–KCl melt16 and from 5 to 5.7 for the KCl–LiCl one.17
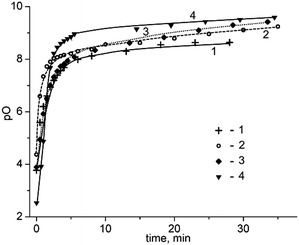 | ||
| Fig. 2 The dependence of pO in the molten KCl–NaCl equimolar mixture vs. time of carbochlorination by the products of CCl4 pyrolysis at 943 (1), 973 (2), 1000 (3) and 1023 K, according to the data.9 | ||
It can be emphasized that in all cases, a monotonous increase of limiting pO value with temperature is observed. This means that the rate of the purification process with the elevation of the temperature rises faster than the rate of the contamination of the melt.
Concerning the possible sources of melt contamination by oxygen-containing admixtures,15 the main factor is the atmosphere over the melt since ceramic construction materials are quite stable at pO values less than 10.
The order, rate constants and activation energies of the carbochlorination process
Quantitative estimations of the carbochlorination process are not so simple. Firstly, this process is heterogeneous and includes several stages. They are: the dissolution of gaseous CCl4 in the melt, its reaction with oxide ions dissolved in the melt and the removal of CO or CO2 from the melt into the gaseous phase.An additional complication is the contamination of the melt by the oxygen-containing admixtures from the crucible, electrodes and argon. Finally, room-temperature kinetic investigations usually imply the creation of certain initial concentrations of the reagents. In the considered case, we cannot saturate the melt with tetrachloromethane and simultaneously prevent its reaction with oxygen-containing admixtures before a certain starting moment. So, the main problem of the study consists of the separation of the chemical stage of this process from other ones and its investigation.
If the chemical reaction (1) is a simple one from the viewpoint of chemical kinetics, then the rate of reaction w can be expressed using the reagent concentrations in such a manner:
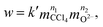 | (9) |
Furthermore, mO2− in the chloride melts decreases until the rates of melt purification and contamination become equal.
Therefore, if we fit the conditions under which the concentration of CCl4 will be practically constant, we will be able to determine the order of this reaction with respect to the oxide ion (pseudo-order) and calculate the corresponding conditional rate constant.
Since the constant partial pressure of tetrachloromethane over the melt is provided in the absence of the carbochlorination process, its concentration in the melt also should be constant according to Henry’s law. This should be true if the consumption of the dissolved CCl4 for the melt treatment is negligible. In practice, this means extremely low oxide ion concentrations. In this case, eqn (9) may be rewritten in the following form to allow estimations:
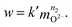 | (10) |
Although the dependences in Fig. 1 and 2 principally resemble typical kinetic plots, one can note an important difference. The experimental dependences are built on a logarithmic scale for the concentration of oxide ions (pO ≡ −0.4343![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mO2−). This fact permits the assumption that the chemical reaction in the melt does not correspond to 1st order kinetics with respect to the oxide ion.
mO2−). This fact permits the assumption that the chemical reaction in the melt does not correspond to 1st order kinetics with respect to the oxide ion.
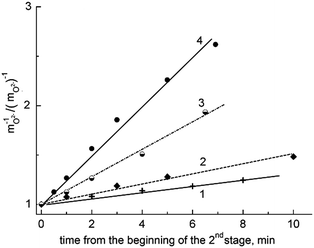
The data15–18 permit the conclusion that the carbochlorination process obeys kinetics of the 2nd order with several peculiarities (see Fig. 3). As is known, for reactions belonging to the 2nd kinetic order, the dependence built in ‘inverse concentration – time’ coordinates should be linear.
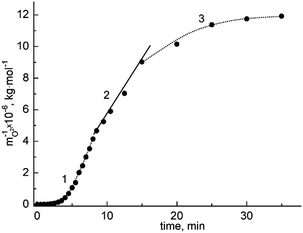 | ||
| Fig. 3 Dependence of mO2−−1 against time for the treatment of the BaCl2–KCl eutectic with CCl4 at 1023 K: (1) – the rate of the process is limited by gas dissolution and ‘BaO–CCl4’ interaction, (2) – the rate of the process is limited by the chemical reaction (1), (3) – a plateau, where the rates of purification and contamination of the melt equalize. | ||
Nevertheless, the plot of inverse concentration vs. time includes an initial non-linear section. As can be seen, the beginning of this section corresponds to the amount of oxide ions (in the form of BaO) being equal to 5 × 10−4 mol kg−1 and the slow rise of mO2−−1 is caused by the relatively small rate of the following interaction:
| CCl4↑ + 2BaO → CO2↑ + 2BaCl2. | (11) |
The second stage corresponds to small oxide ion concentrations, that give the possibility to accept the assumption about there being negligible consumption of the halogenating agent, ascribed to the carbohalogenation process being pseudo-2nd order, i.e., the running of the process obeys 2nd order kinetics with respect to the oxide ions.
Hence, the experimental data belonging to this section can be used for the estimation of some kinetic parameters of the carbochlorination process.
Besides the graphical method, the 2nd order nature of the reaction was confirmed by substitution (integral) and Van’t-Hoff (differential) methods. The treatment by the latter method shows an insignificant deviation of the reaction order from 2.
As is known, the dependence obeying kinetics of the 2nd order is described by the following common equation:
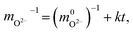 | (12) |
Considering the dependence of the relative inverse concentration of oxide ions against the time of the purification process counted from the beginning of the second stage (Fig. 4), we can note that the increase of temperature results in a monotonous growth of the rate of the chemical reaction (1). The same situation is also observed for other studied chloride melts, agreeing with the general rule of an increase of the reaction rate with temperature.
All of the obtained rate constants of the carbohalogenation process in the chloride melts are collected in Table 1. Using the well-known Arrhenius equation:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = ln k = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − Ea/(RT), A − Ea/(RT),
| (13) |
| Melt | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k k |
Ea, kJ mol−1 | |||
|---|---|---|---|---|---|
| 943 K | 973 K | 1000 K | 1023 K | ||
| KCl–NaCl | 7.30 | 7.39 | 7.83 | 8.12 | 206 ± 30 |
| BaCl2–KCl | 4.79 | 5.03 | 5.43 | 5.82 | 238 ± 30 |
| KCl–LiCl | 3.34 | 3.83 | 4.00 | 4.35 | 177 ± 30 |
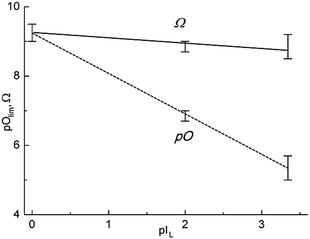
The dependence for pO vs. pIL can be approximated by the following equation:
| pO = 9.34(±0.17) − 1.17(±0.08)pIL, | (14) |
So, using eqn (14) we can calculate the time for purification and the final concentration of oxide ions for an arbitrary melt with known oxoacidic properties.
From common considerations, one can expect that the increase of melt acidity retards the rate of purification, and the stronger the oxoacidic properties of the melt, the higher the residual concentration of oxide ions remaining after the termination of purification. However, the values of the common oxoacidity function of the purified melts, Ω, introduced by Tremillon et al.:18
| Ω = pO + pIL | (15) |
As for the Ω value, it is practically constant and this confirms that for any chloride melt purified under the conditions of our experiments, a common acidity value after purification is dependent, mainly, on the oxoacidic properties of the melt.
So, both the rate and the limit of chloride melt purification decrease with the strengthening of the oxoacidic properties of the melts. The final degree of purification is dependent on the solubility of the constructional materials in the melt and by the content of water and oxygen in the reactive gas atmosphere.
Conclusions
In the frame of this work, some questions connected to kinetic aspects of the carbochlorination of chloride melts by CCl4 vapor are solved. The kinetics and rates of the chemical interaction of oxide ions with CCl4 was estimated to be 2nd order. The chemical stage of melt carbochlorination using CCl4 is characterized by activation energies ca. 200 kJ mol−1 with certain deviations for different melts.The limits of purification for chloride melts with different oxoacidic properties are determined. The obtained plot permits prediction of the main parameters for the deoxidization of an arbitrary melt by such method in the case that its oxoacidic properties are known.
Acknowledgements
This work was supported by the Ministry of Education and Science of Ukraine, project of V. N. Karazin Kharkiv National University No. 16-15-16.Notes and references
- R. O. Suzuki, K. Ono and K. Teranuma, Metall. Mater. Trans. B, 2003, 34, 287 CrossRef.
- A. M. Abdelkader and E. Ek-Kashif, ISIJ Int., 2007, 47, 25 CrossRef CAS.
- A. M. Abdelkader, K. T. Kilby, A. Cox and D. J. Fray, Chem. Rev., 2013, 113, 2863 CrossRef CAS PubMed.
- G. Rooh, H. J. Kim, H. Park and S. Kim, J. Cryst. Growth, 2010, 312, 2243 CrossRef CAS.
- M. Zhuravleva, B. Blalock, K. Yang, M. Koschan and C. L. Melcher, J. Cryst. Growth, 2012, 352, 115 CrossRef CAS.
- A. F. Kuznetsov and V. F. Pekhov, USSR Patent No 201386, 1967.
- Yu. F. Rybkin and O. V. Demirskaya, in Single Crystals and Technics, Inst. Single Crystals, Kharkov, 1974, vol. 1, No 10, p. 115 Search PubMed.
- V. L. Cherginets, O. V. Demirskaya and T. P. Rebrova, Molten Salts Forum, 2000, 7, 163 Search PubMed.
- V. L. Cherginets, T. P. Rebrova, T. V. Ponomarenko, V. A. Naumenko and Y. N. Datsko, RSC Adv., 2014, 4(95), 52915 RSC.
- V. L. Cherginets, Oxoacidity: reactions of oxocompounds in ionic melts, Elsevier, Amsterdam, 2005 Search PubMed.
- Fusion diagrams of salt systems. Part I. Binary systems with common anion [from AgBr-CsBr to In2(WO4)3-Rb2WO4], Handbook, ed. V. I. Posypaiko, et al., Metallurgiya, Moscow, 1977, in Russian Search PubMed.
- Fusion diagrams of salt systems. Part II. Binary systems with common anion [from KBH4-LiBH4 to ZnCl2-ZrCl4], Handbook, ed. V. I. Posypaiko, et al., Metallurgiya, Moscow, 1977, in Russian Search PubMed.
- Y. Castrillejo, M. R. Bermejo, R. Pardo and A. M. Martinez, J. Electroanal. Chem., 2002, 522, 124 CrossRef CAS.
- Y. Castrillejo, M. R. Bermejo, R. Pardo, A. M. Martinez and P. D. Arocas, J. Electroanal. Chem., 2003, 545, 141 CrossRef CAS.
- V. L. Cherginets, T. P. Rebrova, T. V. Ponomarenko, V. A. Naumenko and E. Yu. Bryleva, React. Kinet., Mech. Catal., 2015, 116, 327 CrossRef CAS.
- V. L. Cherginets, T. P. Rebrova, V. A. Naumenko and T. V. Ponomarenko, Phys. Chem. Liq., 2015, 53, 193 CrossRef CAS.
- V. L. Cherginets, V. A. Naumenko, T. V. Ponomarenko and T. P. Rebrova, Problems of Chemistry and Chemical Technology, 2013, 2, 138 Search PubMed.
- R. Combes, B. Tremillon, F. De Andrade, M. Lopes and H. Ferreira, Anal. Lett., 1982, 15, 1585 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |