Green chemistry oriented multi-component strategy to hybrid heterocycles†
Balakrishnan Rajarathinam,
Kandhasamy Kumaravel and
Gnanasambandam Vasuki*
Department of Chemistry, Pondicherry University, R.V. Nagar, Kalapet, Puducherry-605 014, India. E-mail: vasukig@gmail.com; Fax: +91-413-2656740; Tel: +91-413-265498
First published on 27th July 2016
Abstract
An oxindole decorated 4H-chromene scaffold has been synthesized in water under catalyst-free reaction conditions at ambient temperature in good yields with moderate to high diastereoselectivities by implementing an ingenious methodology by integrating the guiding principles of Diversity Oriented Synthesis (DOS) and green chemistry.
Synthetic organic chemistry plays a vital role in medicinal chemistry for identifying new drugs.1 The paucity of potential bioactive scaffolds in modern drug discovery, has challenged synthetic organic chemists to evolve new strategies to synthesize a collection of natural-product-like and non-natural bioactive small molecules.2 Diversity Oriented Synthesis3 (DOS), a splendid area of research in organic synthesis with its sub-disciplines privileged sub-structure based Diversity Oriented Synthesis4 (pDOS) and biology oriented synthesis5 (BIOS) has provided sophisticated ideologies to access bioactive small molecules. In addition, synthetic organic chemists have to abide by the stringent criteria of an ideal organic synthesis.6 Multi-Component Reactions7 (MCRs) in water can be visualized as an “elegant greener approach” and also as cutting-edge research to achieve diverse and complex small organic molecules from simple educts so as to quickly populate the chemical space.
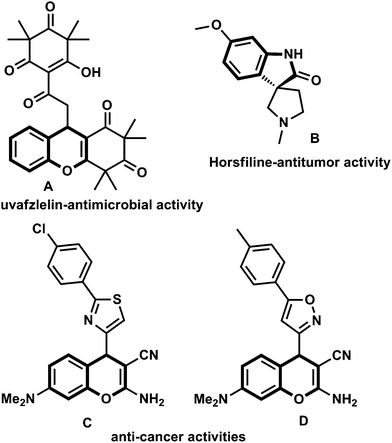
Chromene is one of the privileged pharmacophores8 in medicinal chemistry, which is capable of interacting with a variety of cellular targets and thus possessing a wide range of biological activities9 such as antitumor, antivascular, antimicrobial, antioxidant, antifungal, antiviral, anticancer, anti-HIV, antitubercular and anti-inflammatory activity. In recent decades, chromene derivatives have become attractive motifs as potential anti-cancer agents.10 Oxindole is an another important heterocyclic ring systems found most frequently in small molecule drugs11 and oxindole appended with heterocyclic motifs are ubiquitous in natural products and synthetic bioactive small molecules (Fig. 1).12 Therefore, a hybrid heterocyclic framework consisting of both 4H-chromene and oxindole might possess properties of both and enhance the activity. Synthesizing hybrid heterocyclic frameworks with 4H-chromene and investigating their potential utility as anti-cancer drugs continues to be an active area of research.
To the best of our knowledge only a few reports are available in the literature13 for the synthesis of 4-heterocycle decorated 4H-chromenes-3-carbonitrile derivatives, many of which significantly drift away from green chemistry limitations such as avoidance of organic solvents, harsh reaction conditions, expensive catalysts and prolonged reaction times etc. Performing organic reactions in water, in addition to its eco-benefits, often imparts a remarkable effect on the reactivity and selectivity of organic reactions.14
We are actively engaged in developing multi-component reaction protocols in water with a specific focus of intensifying MCRs in water by integrating the strategies of DOS like Single Reactant Replacement15 (SRR) strategy and BIOS to access biologically relevant small molecules.
In the continued endeavour of our research group to access novel small organic molecules by amalgamating the guiding principles of DOS and green chemistry, a catalyst-free synthesis of 4-pyrazolyl substituted 4H-chromene derivatives16 in water at ambient temperature was achieved as an improvement of our work on accessing 4-azolidinonyl substituted 4H-chromene derivatives in a single operation incorporating stereochemical diversity, we hereby report an efficient and eco-friendly reaction protocol to access this hybrid heterocycle (Scheme 1). A catalyst-free three-component reaction between salicylaldehyde (1), oxindole (2) and malononitrile (3) was performed in water at ambient temperature for 4 h. The product that formed in the reaction was filtered and then washed with ethyl acetate/hexane mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 ratio), which was characterised by IR, 1H, 13C NMR and mass spectral analysis without any further purification. Two methyne protons are observed in 1H NMR spectrum. The coupling constant (J = 2.0–2.8 Hz) of the two vicinal methyne protons (C2 and C3 protons of oxindole and chromene respectively) indicated that the two hydrogens on the chiral carbons have adopted gauche conformation.17 Surprisingly, both diastereomers of S1 (S1a and S1b) had crystallized together.18 Single crystal X-ray diffraction analysis of both S1a and S1b showed that the two hydrogens on C2 and C3 of oxindole and chromene respectively had adopted a gauche conformation (Fig. 3). All these analytical data were supported to an unambiguous confirmation of the product 2-amino-4-(2-oxoindolin-3-yl)-4H-chromene-3-carbonitrile with two contiguous stereocenter. The diastereomers could be separated by simple recrystallization at room temperature, which is shown in Fig. 2.
8 ratio), which was characterised by IR, 1H, 13C NMR and mass spectral analysis without any further purification. Two methyne protons are observed in 1H NMR spectrum. The coupling constant (J = 2.0–2.8 Hz) of the two vicinal methyne protons (C2 and C3 protons of oxindole and chromene respectively) indicated that the two hydrogens on the chiral carbons have adopted gauche conformation.17 Surprisingly, both diastereomers of S1 (S1a and S1b) had crystallized together.18 Single crystal X-ray diffraction analysis of both S1a and S1b showed that the two hydrogens on C2 and C3 of oxindole and chromene respectively had adopted a gauche conformation (Fig. 3). All these analytical data were supported to an unambiguous confirmation of the product 2-amino-4-(2-oxoindolin-3-yl)-4H-chromene-3-carbonitrile with two contiguous stereocenter. The diastereomers could be separated by simple recrystallization at room temperature, which is shown in Fig. 2.
The scope of this tactics was further established with several substituted salicylaldehydes for appendage diversity of the scaffold, though the reaction rate, diastereomer ratio and yield of the product vary with the substituent (Chart 1). The diastereomer ratio for each product was calculated from signal at δ ∼ 3.9–4.3 ppm assignable to the hydrogens at the chiral centers.
 | ||
| Chart 1 2-Amino-4-(2-oxoindolin-3-yl)-4H-chromene-3-carbonitrile S1–S12 (ratio of diastereomers calculated by 1H NMR of isolated product). | ||
The oxindole substituted 4H-chromene hybrid is suggested to occur through (i) condensation of salicylaldehyde with malononitrile followed by intra molecular 6-exo-dig oxa-Thorpe–Ziegler cyclization on nitrile group, to afford 2-imino-3-cyano 2H-chromenes intermediate (4); followed by (ii) Michael addition of 4 with iminol form of oxindole (Scheme 2, path A). The product S1 did not involve in the lactam–lactim tautomerism19 that reflect in the NMR and X-ray analysis.
To verify the mechanism proposed for product formation, a two component reaction between 3-(2-hydroxybenzylidene)indolin-2-one (5) and malononitrile was performed in the optimized reaction condition, but the reaction did not yield oxindole substituted 4H-chromene hybrid (S1) (Scheme 2, path B). Thus, there is a remarkable difference in the nature of products obtained when reactions are performed as conventional stepwise reaction and as a MCR.
Moreover, the nature of product obtained in the MCR and the major tautomer of the azolidinone in the hybrid heterocyclic scaffold is influenced by the nature of the azolidinone active methylene component. While azolidinone, which is more reactive than malononitrile would yield a single chiral center racemic product, a less reactive azolidinone yields a diastereomeric mixture with two contiguous chiral centers. Thus synthesis of 4-azolidinone substituted 4H-chromene hybrid by MCR cannot be considered as an appendage diversity reaction, but installing different biologically important motifs at 4-position of chromene necessitates independent investigations.
Conclusions
A hybrid heterocyclic scaffold, oxindole appended 4H-chromene-3-carbonitrile possessing two biologically active moieties with two contiguous stereo centers has been accessed in good yields by a catalyst-free three-component reaction in water at ambient temperature from simple educts. In addition to the efficiency and environmental benefits, this protocol has provided an insight in the scope of MCR in accessing novel scaffolds. Investigation on the effect of different heterocycles and factors contributing to the reaction to build novel 4-heterocycle substituted 4H-chromene are in progress.Experimental
General procedure for the synthesis of oxindole decorated 4H-chromene scaffold
To a stirred aqueous mixture of oxindole 2 (3 mmol) respective 2-hydroxybenzaldehyde 1 (3 mmol) and malononitrile 3 (3 mmol) were added successively in 25 mL water at ambient temperature under an open atmosphere with vigorous stirring for 4 h. The precipitated solid was filtered, washed with water and then 5 mL of ethyl acetate/hexane mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). The product S1 obtained was pure by TLC and spectral techniques.
8). The product S1 obtained was pure by TLC and spectral techniques.
Acknowledgements
We thank CSIR (01(2500)/11/EMR-II) and DST (SR/S5/GC-22/2007), Government of India, for financial support, Central Instrumentation Facility (CIF), Pondicherry University, for high-resolution NMR and the Department of Chemistry (DST-FIST), Pondicherry University, for FT-IR and single crystal XRD.Notes and references
- M. MacCoss and T. A. Baillie, Science, 2004, 303, 1810 CrossRef CAS PubMed
; Y. Huang, A. Yazbak and A. Dömling, in Green Techniques for Organic Synthesis and Medicinal Chemistry, John Wiley & Sons, Ltd, Chichester, UK, 2012, pp. 497–522 Search PubMed
.
- B. R. Stockwell, Nature, 2004, 432, 846 CrossRef CAS PubMed
; S. L. Schreiber, Science, 2000, 287, 1964 CrossRef PubMed
; B. R. Stockwell and S. L. Schreiber, Curr. Biol., 1998, 8, 761 CrossRef PubMed
; S. L. Schreiber, et al., Nat. Biotechnol., 2010, 28, 904 CrossRef PubMed
.
- M. D. Burke and S. L. Schreiber, Angew. Chem., Int. Ed., 2004, 43, 46 CrossRef CAS PubMed
; R. J. Spandl, A. Bender and D. R. Spring, Org. Biomol. Chem., 2008, 6, 1149 Search PubMed
; S. L. Schreiber, Nature, 2009, 457, 153 CrossRef PubMed
.
- S. Oh and S. B. Park, Chem. Commun., 2011, 47, 12754 RSC
.
- M. A. Koch, A. Schuffenhauer, M. Scheck, S. Wetzel, M. Casaulta, A. Odermatt, P. Ertl and H. Waldmann, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17272 CrossRef CAS PubMed
; R. S. Bon and H. Waldmann, Acc. Chem. Res., 2010, 43, 1103 CrossRef PubMed
; S. Wetzel, R. S. Bon, K. Kumar and H. Waldmann, Angew. Chem., Int. Ed., 2011, 50, 10800 CrossRef PubMed
.
- P. A. Wender and B. L. Miller, Nature, 2009, 460, 197 CrossRef CAS PubMed
.
- J. Zhu and H. Bienaymé, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 CrossRef CAS PubMed
; A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef CAS PubMed
; E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef PubMed
; E. Ruijter and R. V. A. Orru, Drug Discovery Today: Technol., 2013, 10, 15 CrossRef PubMed
; I. V. Magedov and A. Kornienko, Chem. Heterocycl. Compd., 2012, 48, 33 CrossRef
; P. Slobbe, E. Ruijter and R. V. A. Orru, Med. Chem. Commun., 2012, 3, 1189 RSC
; C. Kalinski, M. Umkehrer, L. Weber, J. Kolb, C. Burdack and G. Ross, Mol. Diversity, 2010, 14, 513 CrossRef PubMed
; I. Akritopoulou-Zanze, Curr. Opin. Chem. Biol., 2008, 12, 324 CrossRef PubMed
; H. Eckert, Molecules, 2012, 17, 1074 CrossRef PubMed
.
- G. P. Ellis, The Chemistry of Heterocyclic Compounds: Chromenes, Chromanes and Chromones, ed. A. Weissberger and E. C. Taylor, Wiley, New York, ch. II, 1997 CrossRef CAS PubMed
; R. Pratap and V. J. Ram, Chem. Rev., 2014, 114, 10476 CrossRef CAS PubMed
.
- G. Henriette, L. Lorraine, H. Bettina, D. Clemence, D. Kelly and K. Irenej, et al., Mol. Cancer Ther., 2004, 3, 1375 Search PubMed
; B. S. Chetan, M. S. Nimesh, P. P. Manish and G. P. Ranjan, J. Serb. Chem. Soc., 2012, 77, 1 CrossRef CAS
; M. Milan, M. Mirjana, B. Desanka, M. Sanja, N. Neda and M. Vladimir, et al., Int. J. Mol. Sci., 2011, 12, 2822 CrossRef PubMed
; T. Suresh, V. Arunima, K. Atin, G. Sandeep, V. R. Prarthana and R. K. Ganesh, Acta Pol. Pharm., 2010, 67, 423 Search PubMed
; J. Mori, M. Iwashima, M. Takeuchi and H. A. Saito, Chem. Pharm. Bull., 2006, 54, 391 CrossRef PubMed
; C. K. Denish, K. P. Hetal and K. G. Nilesh, AJBPR, 2012, 2, 126 Search PubMed
; R. K. Nimesh, D. H. Dhaval, T. M. Prashant and K. P. Saurabh, Med. Chem. Res., 2011, 20, 854 CrossRef
; K. Nitin, K. Sushil, G. Himanshu and P. K. Sharma, World Res. J. Biochem., 2012, 1, 1 Search PubMed
.
- M. Vosooghi, S. Rajabalian, M. Sorkhi, M. Badinloo, M. Nakhjiri, A. S. Negahbani, A. Asadipour, M. Mahdavi, A. Shafiee and A. Foroumadi, Res. Pharm. Sci., 2010, 5, 9 Search PubMed
; K. Shailaja, G. Henriette and M. Karen, et al., Mol. Cancer Ther., 2004, 3, 1365 Search PubMed
; M. Mahmoodi, A. Aliabadi, S. Emami, M. Safavi, S. Rajabalian, M. Mohagheghi, A. Khoshzaban, A. Samzadeh-Kermani, N. Lamei, A. Shafiee and A. Foroumadi, Arch. Pharm. Chem. Life Sci., 2010, 343, 411 CrossRef CAS PubMed
; B. Bita, M. H. Majid and A. O. Hossein, J. Korean Chem. Soc., 2009, 53, 631 CrossRef
; T. Akbarzadeh, A. Rafinejad, J. M. Mollaghasem, M. Safavi, A. Fallah-Tafti, M. Pordeli, S. K. Ardestani, A. Shafiee and A. Foroumadi, Arch. Pharm. Chem. Life Sci., 2012, 345, 386 CrossRef PubMed
; N. Thomas and S. M. Zachariah, Asian J. Pharm. Clin. Res., 2013, 6, 11 Search PubMed
.
- R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845 CrossRef CAS PubMed
; Y. Liu, S. Duan, R. Zhang, Y.-H. Liu, J. Chen and W. Xiao, Org. Biomol. Chem., 2016, 14, 5224 Search PubMed
; S. Wang, Y. Jiang, S. Wu, G. Dong, Z. Miao, W. Zhang and C. Sheng, Org. Lett., 2016, 18, 1028 CrossRef PubMed
; L. Zhu, Q. Chen, D. Shen, W. Zhang, C. Shen, X. Zeng and G. Zhong, Org. Lett., 2016, 18, 2387 CrossRef PubMed
; S. Kumari, H. Singh and J. M. Khurana, Tetrahedron Lett., 2016, 57, 3081 CrossRef
.
- P. B. Alper, C. Meyers, A. Lerchner, D. R. Siegel and E. M. Carreira, Angew. Chem., Int. Ed., 1999, 38, 3186 CrossRef CAS
; Y. Cao, X. Jiang, L. Liu, F. Shen, F. Zhang and R. Wang, Angew. Chem., Int. Ed., 2011, 50, 9124 CrossRef PubMed
; T. D. Bagul, G. Lakshmaiah, T. Kawabata and K. Fuji, Org. Lett., 2002, 4, 249 CrossRef PubMed
; C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209 CrossRef
; T. Onishi, P. R. Sebahar and R. M. Williams, Org. Lett., 2003, 5, 3135 CrossRef PubMed
; M. M.-C. Lo, C. S. Neumann, S. Nagayama, E. O. Perlstein and S. L. Schreiber, J. Am. Chem. Soc., 2004, 126, 16077 CrossRef PubMed
; K. Ding, Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey, K. Krajewski, P. P. Roller and S. Wang, J. Med. Chem., 2006, 49, 3432 CrossRef PubMed
; C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef PubMed
; B. M. Trost and D. T. Stiles, Org. Lett., 2007, 9, 2763 CrossRef PubMed
.
- M. N. Elinson, A. S. Dorofeev, F. M. Miloserdov, A. I. Ilovaisky, S. K. Feducovich, P. A. Belyakov and G. I. Nikishina, Adv. Synth. Catal., 2008, 350, 591 CrossRef CAS
; N. V. Lakshmi, S. E. Kiruthika and P. T. Perumal, Synlett, 2011, 1389 Search PubMed
.
- Organic Reactions in Water: Principles, Strategies and Applications, ed. U. M. Lindström, Blackwell publishing, Oxford, UK, 2007 CrossRef CAS PubMed
; L. Weber, Drug Discovery Today, 2002, 7, 143 CrossRef CAS PubMed
; C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51 CrossRef PubMed
; A. Domling, Chem. Rev., 2006, 106, 17 CrossRef PubMed
; I. Kanizsai, S. Gyonfalvi, Z. Szadonyi, R. Sillanpaa and F. Fulop, Green Chem., 2007, 9, 357 RSC
; K. Kumaravel and G. Vasuki, Curr. Org. Chem., 2009, 13, 1820 CrossRef
; Y. Gu, Green Chem., 2012, 14, 2091 RSC
; M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC
.
- B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef CAS PubMed
.
- K. Kumaravel and G. Vasuki, Green Chem., 2009, 11, 1945 RSC
.
- G. Bifulco, P. Dambruoso, L. Gomez-Paloma and R. Riccio, Chem. Rev., 2007, 107, 3744 CrossRef CAS PubMed
.
- CCDC 1010756 (S1), 1010757 (S4) and 1010767 (S7).†.
- C. S. Peng and A. Tokmakoff, J. Phys. Chem. Lett., 2012, 3, 3302 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 13C NMR spectra for all reaction products are available. CCDC 1010756, 1010757 and 1010767. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra11543h |
| This journal is © The Royal Society of Chemistry 2016 |