The synthesis of iminothiophenone-fused quinolines and evaluation of their serendipitous reactions†
Abstract
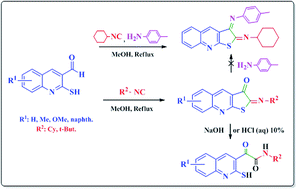
The novel synthesis of tricyclic 2-(cyclohexylimino)thieno[2,3-b]quinolin-3(2H)-ones from the reaction of 2-mercaptoquinoline-3-carbaldehydes and isocyanides in methanol without the use of any additive is described. This protocol proceeds with high atom economy through the formation of C–S and C–C bonds and then oxidation via tandem reaction. Moreover, some other remarkable aspects of this reaction, such as the hydrolysis and three-component reaction with the aromatic amine to yield a highly conjugated Schiff base, are also investigated.

 Please wait while we load your content...
Please wait while we load your content...