Photoisomerization and optical properties of a subphthalocyanine–azobenzene–subphthalocyanine triad†
Maohu Shia,
Yue Zhaoa,
Haijun Xub,
John Mack*c,
Luan Yind,
Xiaoyong Wangd and
Zhen Shen*a
aState Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Nanjing 210046, P. R. China. E-mail: zshen@nju.edu.cn; Fax: +86-25-8968-2309; Tel: +86-25-8968-6679
bCollege of Chemical Engineering, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, Nanjing Forestry University, Nanjing 210037, P. R. China
cDepartment of Chemistry, Rhodes University, Grahamstown 6140, South Africa. E-mail: j.mack@ru.ac.za; Fax: +27-46-622-5109; Tel: +27-46-603-7234
dNational Laboratory of Solid State Microstructures, School of Physics, Nanjing University, Nanjing 210093, P. R. China
First published on 20th July 2016
Abstract
The synthesis and characterization of a subphthalocyanine–azobenzene–subphthalocyanine triad is reported. Evidence for trans ↔ cis isomerization of the linking azobenzene moiety is observed in the NMR and optical spectra when the subphthalocyanine rings are used as light-harvesting units. Significantly, a decrease in fluorescence intensity is observed on moving from trans → cis with a recovery in intensity observed on moving back from cis → trans that can be attributed to a change in the rate of non-radiative decay.
Introduction
It is well known that azobenzene (AB) exhibits a reversible trans ↔ cis isomerization upon irradiation with UV and visible region light. It has previously been demonstrated that the AB moiety can be incorporated as a light triggered molecular switch into a variety of different structures, and numerous multi-functional materials have been synthesized and investigated for use in applications on this basis.1 Heteroaromatic macrocycles, such as phthalocyanines and porphyrins,2 show distinctive properties when they are incorporated into AB structures. For example, an AB-substituted zinc phthalocyanine exhibits photoresponsive J-aggregation behaviour,3 and the isomerization behaviour of an azo-based silicon phthalocyanine photoswitch can be modulated upon the absorption of an incident photon by the phthalocyanine ring.4 It has also been demonstrated that reversible isomerization of two axial AB ligands leads to the modulation of the fluorescence of phosphorus(V) porphyrin5 and similar properties have been reported for an AB-linked zinc diporphyrin.6Porphyrin and fullerene moieties have been linked by an AB moiety to form a donor–acceptor system,7 and a similar triad was formed with a bowl-shaped subphthalocyanine (subPc) replacing the porphyrin as the donor group.8 SubPcs9 are comprised of three isoindoline moieties coordinating a tetrahedral boron atom with an axial ligand, linked by aza-nitrogen atoms (Scheme 1). Intense absorption (550–600 nm) and emission are observed in the visible region due to the heteroaromatic 14 π-electron system, which can act as an efficient light-harvesting unit.10 Intense luminescence is usually observed for subPcs, since there is little or no aggregation in solution due to the cone-shaped structure of the subPc ligand. Due to easy axial ligand exchange,11 multi-functional subPcs can be readily prepared and their possible utility for use as organic photodetectors,12 and in molecular cages,13 organic solar cells14 and organic light-emitting diodes (OLEDs),15 and also in photodynamic therapy (PDT)16 and as sensor probes,17 has been studied. Herein the synthesis and characterization of an axially conjugated subPc–AB–subPc triad (1) is reported, along with its trans ↔ cis photoisomerization properties. 1 has been fully characterized by 1H, 13C and 11B NMR, HR-MS, X-ray single crystal structure analysis, UV-visible absorption and fluorescence spectroscopy.
Experimental
Materials and instrumentation
All reagents were obtained from commercial suppliers and used without further purification unless otherwise indicated. Air and moisture-sensitive reactions were carried out under an argon atmosphere. HR-MS data were collected using an LTQ-Orbitrap mass spectrometer XL MS (Thermofisher, Bremen, Germany) equipped with an electrospray ionization interface (ESI). The NMR spectroscopic measurements were recorded on a Bruker 500 or 400 MHz spectrometer with CDCl3 or dimethyl sulfoxide-d6 as the solvent.Synthesis of 1
2 (86 mg, 0.2 mmol, 2 equiv.) was added to a solution of 3 (21.4 mg, 0.1 mmol, 1 equiv.) in dry toluene held in a 15 ml pressure vessel, and the mixture was heated at 180 °C for 5 days. The solvent was evaporated under reduced pressure. The crude products were purified by neutral alumina column chromatography with THF as the eluent to obtain a cardinal red solid (26 mg, yield: 26%). 1H NMR (400 MHz, CDCl3) δ 8.85–8.87 (m, 12H), 7.90–7.93 (m, 12H), 7.23 (d, J = 8.8 Hz, 4H), 5.41 (d, J = 8.8 Hz, 4H). 13C NMR (100 MHz, CDCl3) δ 151.36, 130.94, 130.16, 129.92, 123.61, 122.41, 122.28, 119.18. 11B NMR (400 MHz, CDCl3) δ −14.81. HR-MS m/z: calcd for C60H32B2N14O2: 1002.3019, found: 1003.3223 [M + H]+.X-ray structure determination
The X-ray crystallographic data for 1 was carried out at 291 K on a Rigaku Saturn CCD spectrometer with graphite monochromated Mo Kα radiation (λ = 0.71070 Å). The structure was solved by direct methods and refined on F2 by full-matrix least-squares using the Crystal Clear and (SHELXS-97) programs. 1: C60H32B2N14O2; a yellow block-like crystal of approximate 0.27 × 0.25 × 0.22 mm3 dimensions was measured. Space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.671(3) Å, b = 11.787(3) Å, c = 12.167(3) Å, α = 90.260(4)°, β = 94.290(5)°, γ = 105.427(4)°, V = 1470.6(7) Å3, Z = 1, F(000) = 516, ρ = 1.13204 g cm−3, R1 = 0.2085, wR2 = 0.2973, GOF = 1.003. CCDC 1401329 contains the supplementary crystallographic data for this paper.†
, a = 10.671(3) Å, b = 11.787(3) Å, c = 12.167(3) Å, α = 90.260(4)°, β = 94.290(5)°, γ = 105.427(4)°, V = 1470.6(7) Å3, Z = 1, F(000) = 516, ρ = 1.13204 g cm−3, R1 = 0.2085, wR2 = 0.2973, GOF = 1.003. CCDC 1401329 contains the supplementary crystallographic data for this paper.†
Spectroscopic measurements
UV-visible absorption spectra were recorded with a Shimadzu UV-2550 spectrophotometer. Fluorescence measurements were carried out on a Hitachi F-4600 spectrofluorometer. Solutions for absorption and emission measurements were contained in 1 × 1 cm quartz cuvettes. For all measurements, the temperature was kept constant at 298 ± 2 K. Fluorescence lifetimes were measured using a time correlated single photon counting (TCSPC) setup (Single Photon Avalanche Diodes, PDM series, Picoquant GmbH). The excitation source was a diode laser (LDH-P-C-405 with 10 MHz repetition rate, 70 ps pulse width).Computational details
Optimized geometries for the trans and cis conformers of 1 (trans-1 and cis-1) and the corresponding AB structures were obtained by using the B3LYP functional of the Gaussian 09 software package18 with 6-31G(d) basis sets in DMSO using the polarized continuum model with the default settings. TD-DFT calculations for B3LYP optimized geometries for the trans-1 and cis-1 carried out in a similar manner at the LC-ωPBE/6-31G(d) and B3LYP/6-31G(d) levels of theory.Results and discussion
Synthesis
The synthetic strategy for the subphthalocyanine–azobenzene–subphthalocyanine triad 1 is shown in Scheme 1. Boron subphthalocyanine chloride (2)19 and 4,4′-bis-hydroxyazobenzene (3)20 were prepared according to the reported procedures (the details are provided as ESI†). 1 was synthesized from 2 and 3 by using dry toluene as the solvent, and was obtained in 26% yield after purification by column chromatography.Crystal structure
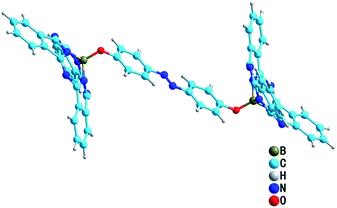
Single crystals of 1 were obtained through the slow diffusion of methanol into a chloroform solution. The crystal structure of 1 is shown in Fig. 1. As has been reported previously for a wide range of subPcs,9 the boron atom is coordinated in a tetrahedral geometry by three nitrogen and one oxygen atoms, resulting in a bowl-shaped conical conformation for the two subPc moieties that form an opposite configuration. The azobenzene moiety is highly planar in trans form, in which bond lengths of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N are 1.134 Å and 1.512 Å respectively. And the B–O–C angle is 117.8°.
N and C–N are 1.134 Å and 1.512 Å respectively. And the B–O–C angle is 117.8°.
Spectroscopic properties of the photoisomerization process
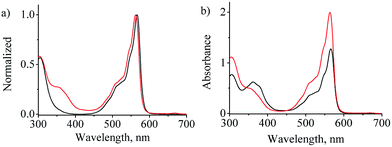
In almost all cases, the boron atom gives rise to a sharp singlet NMR peak that lies between −13.8 and −19.6 ppm.9,21 The observed peak at −14.81 ppm confirms that the boron atoms are tetra substituted (the details are provided as ESI†). The UV-visible absorption spectrum of triad 1 in DMF is similar to that of (Cl)BsubPc (2) spectral profile with a B band at ca. 300 nm and a Q band in the 550–600 nm region (Fig. 2). A shoulder of intensity corresponding to the main azobenzene band, is observed in the 357 nm region. The spectrum of 1 does not correspond to a summation of the spectra of 2 and 3 in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of 1 (Fig. 2), since intramolecular interactions result in an increase in the intensity for the B and Q bands of the subPc moieties and a decrease in the intensity of the 357 nm band of the AB moiety. This provides clear evidence for the formation of the triad.
1 stoichiometry of 1 (Fig. 2), since intramolecular interactions result in an increase in the intensity for the B and Q bands of the subPc moieties and a decrease in the intensity of the 357 nm band of the AB moiety. This provides clear evidence for the formation of the triad.
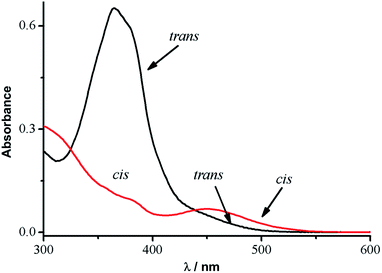
The photoisomerization of 1 was studied in dilute DMF solutions. Conversion of the trans conformer to a cis rich photostationary state (PSS) was accomplished through irradiation with 365 nm light. The cis → trans back reaction was achieved in the visible region with a 570 nm laser, indicating that subPc can act as a light-harvesting unit in this context. In the dark, the isomerization occurs or followed under about two days dark conditions. A shoulder of intensity is observed at 357 nm (Fig. 3), which can be assigned to the main trans-AB π–π* transition, since this band loses intensity upon irradiation at 365 nm. The photoisomerization of AB 3 was studied using dilute DMSO solutions and significant spectral changes were observed (Fig. 4), which are consistent with this band assignment. Upon irradiation at 365 nm, 3 undergoes trans → cis photoisomerization, while the cis → trans back reaction was achieved through irradiation at 490 nm. After several reversible photoisomerization cycles,22 only limited photochemical degradation of 1 occurs when spectral changes are monitored at 357 nm (Fig. 3).23 This means that 1 meets the basic requirements for use as a molecular photoswitch.
TD-DFT calculations were carried out for B3LYP optimized structures for trans-1 and cis-1 at the LC-ωPBE/6-31G(d) and B3LYP/6-31G(d) levels of theory (Fig. 5 and S6†). The B3LYP functional is known to significantly underestimate the energies of transitions with significant charge transfer character,24 so calculations should be carried out with more than one functional to check the validity of band assignments. The spectra of the trans and cis conformers of most ABs contain weak forbidden n–π* bands at ca. 450 nm,23 and this transition is predicted to lie slightly to the blue of the subPc Q band in the spectra of cis-1 and trans-1 in the envelope of weaker vibrational bands (Fig. 5 and S6†). The Q and B bands of the subPc moiety are predicted to be almost unaffected by the change in the conformation of the AB linker moiety, as is observed experimentally (Fig. 3) and lie in the spectral regions characteristic for these transitions in subPc spectra,9,25 so can be assigned unambiguously based on the spectroscopic measurements. The main π–π* bands of cis-ABs typically lie in the 240–280 nm region, while those of trans-ABs lie at ca. 320 nm.26 The TD-DFT calculations provide further evidence that the shoulder of intensity at 357 nm can be assigned to the trans-1 structure on this basis (Fig. 5 and S6†). The marked shift of this band to significantly higher energy in the spectrum of cis-1 (Fig. 5) explains the most significant spectral change that is observed on photoisomerization (Fig. 4).
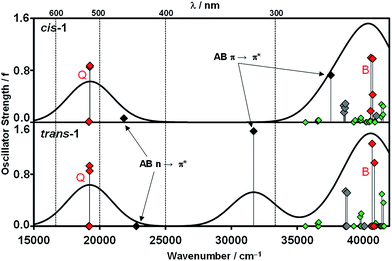 | ||
| Fig. 5 TD-DFT calculations for the B3LYP optimized geometries of trans-1 and cis-1 carried out at the LC-ωPBE/6-31G(d) level of theory in DMSO. The Q and B bands of the subPc moiety are highlighted with red diamonds, while green and black diamonds are used for bands associated with the subPc rings and AB linker, respectively. Gray diamonds denote charge transfer bands. The Chemcraft program34 was used to generate simulated spectra with a band width at half height of 4000 cm−1. Spectra calculated at the B3LYP/6-31G(d) level of theory are provided as ESI.† | ||
Triad 1 exhibits fluorescence from the S1 state of the subPc moiety, due to deactivation of the AB excited state through the isomerization process.27 Upon excitation at λ = 400 nm, a fluorescence emission band is observed at 587 nm. The decay curve can be fitted with a single exponential decay curve that has a lifetime (τF) of 2.52 ns (Fig. 6), which is close to the value reported for subPc 2.28 A decrease in emission intensity is observed after trans → cis isomerization upon irradiation at 365 nm, while there is a recovery in the observed intensity upon irradiation at 570 nm after cis → trans isomerization (Fig. 6). In the absence of aggregation-induced emission,29 dative bonding interactions,30 hydrogen bonding27 and metal binding,31 and the minor fluorescence changes that have been attributed to steric repulsion32 in AB derivatives, the most obvious explanation is a change in the rate of non-radiative decay. The AB moiety of 1 adopts a planar conformation in the trans conformer (Fig. 1). When isomerization takes place to form the cis conformer, there is greater scope for conformational flexibility based on changes to the dihedral angle around the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (Fig. 7), since the AB moiety no longer has a planar π-conjugation system. Conformational flexibility usually results in an enhanced rate of non-radiative decay and hence a decrease in emission intensity. In DMSO, the energy gap between the B3LYP optimized structures of the cis- and trans-1 structures was found to be 14.6 kcal mol−1, only slightly higher than the value of 13.5 kcal mol−1 obtained for the parent AB structures. When structures were optimized with one of the subPc rings aligned in the opposing orientation with respect to the B–O bonds from those shown in Fig. 7, there were negligible differences in the energies of the B3LYP optimized structures and in the predicted optical properties (the details are provided as ESI†).
N bond (Fig. 7), since the AB moiety no longer has a planar π-conjugation system. Conformational flexibility usually results in an enhanced rate of non-radiative decay and hence a decrease in emission intensity. In DMSO, the energy gap between the B3LYP optimized structures of the cis- and trans-1 structures was found to be 14.6 kcal mol−1, only slightly higher than the value of 13.5 kcal mol−1 obtained for the parent AB structures. When structures were optimized with one of the subPc rings aligned in the opposing orientation with respect to the B–O bonds from those shown in Fig. 7, there were negligible differences in the energies of the B3LYP optimized structures and in the predicted optical properties (the details are provided as ESI†).
 | ||
| Fig. 7 The B3LYP optimized geometries for the trans and cis conformers of 1. The trans-1 structure is almost identical to the X-ray structure in Fig. 1. | ||
NMR changes in photoisomerization process
NMR spectroscopy can also be used to investigate the isomerization process.22d,33 The 2D 1H–1H COSY spectrum of 1 (Fig. 8) contains four peaks centered at 8.8, 7.9 ppm, 7.2 and 5.4 ppm. The two upfield peaks that coupled to each other can be readily assigned to the protons from AB linker since they are shielded by the aromatic ring current of two neighbouring subPcs, while two downfield signals are protons from subPcs. When a CDCl3 solution of 1 is irradiated with UV region light (365 nm), two new peaks are observed at 5.24 (d, J = 8.8 Hz) and 6.15 (d, J = 8.7 Hz), and the doublet subPc proton peak at 8.80 ppm gradually change into multiple peaks, indicating that trans → cis conformation change cause the proton signals of AB linker further upfield shifted. This is consistent with the optimized geometry of cis conformer in which two subPc moieties are closer than in the trans conformer (Fig. 7). Integration of the 1H NMR spectra indicates that 25% of triad 1 is present as the cis form in the PSS that is formed in this context. The appearance of the new NMR peaks indicates that there is a drastic conformational change upon isomerization. | ||
| Fig. 8 (a) The 2D 1H–1H COSY spectrum of 1 in CDCl3. (b) 1H NMR spectra of 1 in CDCl3: (b1) before photoirradiation, (b2) irradiation at 365 nm for 1.5 h, (b3) for 5.5 h, (b4) for 7.5 h. | ||
Conclusions
In conclusion, a novel subPc–AB–subPc triad has been synthesized and fully characterized. Evidence for multiple reversible trans ↔ cis isomerization cycles was obtained from the spectral changes that are associated primarily with the AB linker moiety. Additional evidence for the conformational change can be deduced from other minor spectral changes that are observed in the NMR spectroscopy. Significantly, a decrease in fluorescence intensity is observed on moving from trans → cis with a recovery in intensity observed on moving back from cis → trans that can be attributed to changes in the rate of non-radiative decay. Efforts to explore the suitability of other novel multi-functional subPc triads and their suitability for use as light-sensitive or photochromic materials are currently underway.Acknowledgements
Financial support was provided by the Major State Basic Research Development Program of China (Grant No. 2013CB922101 & 2011CB808704), the National Natural Science Foundation of China (No. 21371090), the Natural Science Foundation of Jiangsu Province (BK20130054) to ZS and the China-South Africa joint research program (CS08-L07 and UID: 95421) to ZS and JM, and an NRF of South Africa CSUR grant (93627) to JM.Notes and references
- (a) O. Nachtigall, C. Kördel, L. H. Urner and R. Haag, Angew. Chem., Int. Ed., 2014, 53, 9669–9673 CrossRef CAS PubMed; (b) C. Hoppmann, P. Schmieder, P. Domaing, G. Vogelreiter, J. Eichhorst, B. Wiesner, I. Morano, K. Rück-Braun and M. Beyermann, Angew. Chem., Int. Ed., 2011, 50, 7699–7702 CrossRef CAS PubMed; (c) D. Bleger, Z. Yu and S. Hecht, Chem. Commun., 2011, 47, 12260–12266 RSC; (d) A. A. Beharry and G. A. Woolley, Chem. Soc. Rev., 2011, 40, 4422–4437 RSC; (e) A. A. Beharry, L. Wong, V. Tropepe and G. A. Woolley, Angew. Chem., Int. Ed., 2011, 50, 1325–1327 CrossRef CAS PubMed; (f) M.-M. Russew and S. Hecht, Adv. Mater., 2010, 22, 3348–3360 CrossRef CAS PubMed; (g) O. Sadovski, A. A. Beharry, F. Zhang and G. A. Woolley, Angew. Chem., Int. Ed., 2009, 48, 1484–1486 CrossRef CAS PubMed; (h) Y. Kim, J. A. Phillips, H. Liu, H. Kang and W. Tan, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6489–6494 CrossRef CAS PubMed; (i) M. Yamada, M. Kondo, J.-i. Mamiya, Y. Yu, M. Kinoshita, C. J. Barrett and T. Ikeda, Angew. Chem., Int. Ed., 2008, 47, 4986–4988 CrossRef CAS PubMed; (j) P. Gorostiza and E. Y. Isacoff, Science, 2008, 322, 395–399 CrossRef CAS PubMed; (k) E. R. Kay, D. A. Leigh and F. Zerbetto, Angew. Chem., Int. Ed., 2007, 46, 72–191 CrossRef CAS PubMed; (l) A. Fihey, A. Perrier, W. R. Browne and D. Jacquemin, Chem. Soc. Rev., 2015, 44, 3719–3759 RSC.
- (a) L. J. Esdaile, P. Jensen, J. C. McMurtrie and D. P. Arnold, Angew. Chem., Int. Ed., 2007, 46, 2090–2093 CrossRef CAS PubMed; (b) T. Muraoka, K. Kinbara and T. Aida, Nature, 2006, 440, 512–515 CrossRef CAS PubMed.
- (a) X. Zhou, Z. Chen, Y. Wang, Y. Guo, C.-H. Tung, F. Zhang and X. Liu, Chem. Commun., 2013, 49, 10614–10616 RSC; (b) Z. Chen, C. Zhong, Z. Zhang, Z. Li, L. Niu, Y. Bin and F. Zhang, J. Phys. Chem. B, 2008, 112, 7387–7394 CrossRef CAS PubMed.
- J. L. Rodriguez-Redondo, A. Sastre-Santos, F. Fernandez-Lazaro, D. Soares, G. C. Azzellini, B. Elliott and L. Echegoyen, Chem. Commun., 2006, 1265–1267 RSC.
- (a) D. R. Reddy and B. G. Maiya, J. Phys. Chem. A, 2003, 107, 6326–6333 CrossRef CAS; (b) D. R. Reddy and B. G. Maiya, Chem. Commun., 2001, 117–118 RSC.
- S. Tsuchiya, J. Am. Chem. Soc., 1998, 121, 48–53 CrossRef.
- D. I. Schuster, K. Li, D. M. Guldi, A. Palkar, L. Echegoyen, C. Stanisky, R. J. Cross, M. Niemi, N. V. Tkachenko and H. Lemmetyinen, J. Am. Chem. Soc., 2007, 129, 15973–15982 CrossRef CAS PubMed.
- J.-H. Kim, M. E. El-Khouly, Y. Araki, O. Ito and K.-Y. Kay, Chem. Lett., 2008, 37, 544–545 CrossRef CAS.
- C. G. Claessens, D. González-Rodríguez, M. S. Rodríguez-Morgade, A. Medina and T. Torres, Chem. Rev., 2013, 114, 2192–2277 CrossRef PubMed.
- (a) C. Romero-Nieto, A. Medina, A. Molina-Ontoria, C. G. Claessens, L. Echegoyen, N. Martin, T. Torres and D. M. Guldi, Chem. Commun., 2012, 48, 4953–4955 RSC; (b) M. E. El-Khouly, J.-H. Kim, J.-H. Kim, K.-Y. Kay and S. Fukuzumi, J. Phys. Chem. C, 2012, 116, 19709–19717 CrossRef CAS.
- J. Guilleme, L. Martínez-Fernández, D. González-Rodríguez, I. Corral, M. Yáñez and T. Torres, J. Am. Chem. Soc., 2014, 136, 14289–14298 CrossRef CAS PubMed.
- (a) K.-H. Lee, G. H. Lee, D.-S. Leem, J. Lee, J. W. Chung, X. Bulliard, H. Choi, K.-B. Park, K.-S. Kim, Y. W. Jin, S. Lee and S. Y. Park, J. Phys. Chem. C, 2014, 118, 13424–13431 CrossRef CAS; (b) K.-H. Lee, D.-S. Leem, J. S. Castrucci, K.-B. Park, X. Bulliard, K.-S. Kim, Y. W. Jin, S. Lee, T. P. Bender and S. Y. Park, ACS Appl. Mater. Interfaces, 2013, 5, 13089–13095 CrossRef CAS PubMed.
- (a) C. G. Claessens and T. Torres, Chem. Commun., 2004, 1298–1299 RSC; (b) C. G. Claessens and T. Torres, J. Am. Chem. Soc., 2002, 124, 14522–14523 CrossRef CAS PubMed.
- (a) S. M. Menke, W. A. Luhman and R. J. Holmes, Nat. Mater., 2013, 12, 152–157 CrossRef CAS PubMed; (b) R. Pandey, A. A. Gunawan, K. A. Mkhoyan and R. J. Holmes, Adv. Funct. Mater., 2012, 22, 617–624 CrossRef CAS; (c) A. W. Hains, Z. Liang, M. A. Woodhouse and B. A. Gregg, Chem. Rev., 2010, 110, 6689–6735 CrossRef CAS PubMed; (d) P. Heremans, D. Cheyns and B. P. Rand, Acc. Chem. Res., 2009, 42, 1740–1747 CrossRef CAS PubMed; (e) H. H. P. Gommans, D. Cheyns, T. Aernouts, C. Girotto, J. Poortmans and P. Heremans, Adv. Funct. Mater., 2007, 17, 2653–2658 CrossRef CAS; (f) K. L. Mutolo, E. I. Mayo, B. P. Rand, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2006, 128, 8108–8109 CrossRef CAS PubMed.
- (a) G. E. Morse, M. G. Helander, J. F. Maka, Z.-H. Lu and T. P. Bender, ACS Appl. Mater. Interfaces, 2010, 2, 1934–1944 CrossRef CAS; (b) M. G. Helander, G. E. Morse, J. Qiu, J. S. Castrucci, T. P. Bender and Z.-H. Lu, ACS Appl. Mater. Interfaces, 2010, 2, 3147–3152 CrossRef CAS PubMed.
- (a) M. B. Spesia and E. N. Durantini, Dyes Pigm., 2008, 77, 229–237 CrossRef CAS; (b) H. Xu, X.-J. Jiang, E. Y. M. Chan, W.-P. Fong and D. K. P. Ng, Org. Biomol. Chem., 2007, 5, 3987–3992 RSC.
- (a) M. Ince, N. Gartmann, C. G. Claessens, T. s. Torres and D. Brühwiler, Org. Lett., 2011, 13, 4918–4921 CrossRef CAS PubMed; (b) K. Adachi and H. Watarai, Anal. Chem., 2006, 78, 6840–6846 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- C. G. Claessens, D. González-Rodríguez, B. del Rey, T. Torres, G. Mark, H.-P. Schuchmann, C. von Sonntag, J. G. MacDonald and R. S. Nohr, Eur. J. Org. Chem., 2003, 2003, 2547–2551 CrossRef.
- (a) S. Chakraborty, L. Rajput and G. R. Desiraju, Cryst. Growth Des., 2014, 14, 2571–2577 CrossRef CAS; (b) L. Zhu, H. Yan, K. T. Nguyen, H. Tian and Y. Zhao, Chem. Commun., 2012, 48, 4290–4292 RSC; (c) C. Kordel, C. S. Popeney and R. Haag, Chem. Commun., 2011, 47, 6584–6586 RSC; (d) X. Ma, Q. Wang, D. Qu, Y. Xu, F. Ji and H. Tian, Adv. Funct. Mater., 2007, 17, 829–837 CrossRef CAS.
- (a) J. Guilleme, D. González-Rodríguez and T. Torres, Angew. Chem., Int. Ed., 2011, 50, 3506–3509 CrossRef CAS PubMed; (b) M. S. Rodríguez-Morgade, C. G. Claessens, A. Medina, D. González-Rodríguez, E. Gutiérrez-Puebla, A. Monge, I. Alkorta, J. Elguero and T. Torres, Chem.–Eur. J., 2008, 14, 1342–1350 CrossRef PubMed.
- (a) Y. Yang, R. P. Hughes and I. Aprahamian, J. Am. Chem. Soc., 2014, 136, 13190–13193 CrossRef CAS PubMed; (b) S. Samanta, A. A. Beharry, O. Sadovski, T. M. McCormick, A. Babalhavaeji, V. Tropepe and G. A. Woolley, J. Am. Chem. Soc., 2013, 135, 9777–9784 CrossRef CAS PubMed; (c) Y. Yang, R. P. Hughes and I. Aprahamian, J. Am. Chem. Soc., 2012, 134, 15221–15224 CrossRef CAS PubMed; (d) A. A. Beharry, O. Sadovski and G. A. Woolley, J. Am. Chem. Soc., 2011, 133, 19684–19687 CrossRef CAS PubMed.
- R. Siewertsen, H. Neumann, B. Buchheim-Stehn, R. Herges, C. Näther, F. Renth and F. Temps, J. Am. Chem. Soc., 2009, 131, 15594–15595 CrossRef CAS PubMed.
- T. Stein, L. Kronik and R. Baer, J. Am. Chem. Soc., 2009, 131, 2818–2820 CrossRef CAS PubMed.
- J. Mack, T. Otaki, W. S. Durfee, N. Kobayashi and M. J. Stillman, J. Inorg. Biochem., 2014, 136, 122–129 CrossRef CAS PubMed.
- H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- H. M. D. Bandara, T. R. Friss, M. M. Enriquez, W. Isley, C. Incarvito, H. A. Frank, J. Gascon and S. C. Burdette, J. Org. Chem., 2010, 75, 4817–4827 CrossRef CAS PubMed.
- P. V. Solntsev, K. L. Spurgin, J. R. Sabin, A. A. Heikal and V. N. Nemykin, Inorg. Chem., 2012, 51, 6537–6547 CrossRef CAS PubMed.
- M. Han and M. Hara, J. Am. Chem. Soc., 2005, 127, 10951–10955 CrossRef CAS PubMed.
- (a) J. Yoshino, A. Furuta, T. Kambe, H. Itoi, N. Kano, T. Kawashima, Y. Ito and M. Asashima, Chem.–Eur. J., 2010, 16, 5026–5035 CrossRef CAS PubMed; (b) J. Yoshino, N. Kano and T. Kawashima, Chem. Commun., 2007, 559–561 RSC.
- (a) I. Aiello, M. Ghedini and M. La Deda, J. Lumin., 2002, 96, 249–259 CrossRef CAS; (b) M. Ghedini, D. Pucci, A. Crispini, I. Aiello, F. Barigelletti, A. Gessi and O. Francescangeli, Appl. Organomet. Chem., 1999, 13, 565–581 CrossRef CAS; (c) Y. Wakatsuki, H. Yamazaki, P. A. Grutsch, M. Santhanam and C. Kutal, J. Am. Chem. Soc., 1985, 107, 8153–8159 CrossRef CAS.
- M. Han, D. Ishikawa, E. Muto and M. Hara, J. Lumin., 2009, 129, 1163–1168 CrossRef CAS.
- (a) S. Bellotto, S. Chen, I. Rentero Rebollo, H. A. Wegner and C. Heinis, J. Am. Chem. Soc., 2014, 136, 5880–5883 CrossRef CAS PubMed; (b) T. Ogoshi, K. Kida and T.-a. Yamagishi, J. Am. Chem. Soc., 2012, 134, 20146–20150 CrossRef CAS PubMed; (c) S. Venkataramani, U. Jana, M. Dommaschk, F. D. Sönnichsen, F. Tuczek and R. Herges, Science, 2011, 331, 445–448 CrossRef CAS PubMed; (d) Y. Norikane, R. Katoh and N. Tamaoki, Chem. Commun., 2008, 1898–1900 RSC.
- G. A. Andrienko, Chemcraft version 1.8, http://www.chemcraftprog.com Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray analysis of 1, synthesis details, NMR spectra, HR-MS data and additional TD-DFT calculations. CCDC 1401329. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra11452k |
| This journal is © The Royal Society of Chemistry 2016 |