Lecanicillones A–C, three dimeric isomers of spiciferone A with a cyclobutane ring from an entomopathogenic fungus Lecanicillium sp. PR-M-3†
Zai-Ying Wanga,
Xia-Nan Sanga,
Ke Sunb,
Sheng-Dong Huanga,
Sheng-Shuang Chena,
Chun-Mei Xuea,
Lan-Feng Banb,
Zhan-Lin Lia,
Hui-Ming Huaa,
Yue-Hu Peia and
Jiao Bai*a
aKey Laboratory of Structure-Based Drug Design & Discovery Ministry of Education, Shenyang Pharmaceutical University, Shenyang, 110016, Liaoning, P. R. China
bState Key Laboratory of the Discovery and Development of Novel Pesticide, Shenyang Research Institute of Chemical Industry Co., Ltd, Shenyang 110021, Liaoning, P. R. China
First published on 24th August 2016
Abstract
Lecanicillones A–C (1–3), three unusual dimeric spiciferones with acyclobutane ring via a [2 + 2] cycloaddition, were isolated from an entomopathogenic fungus Lecanicillium sp. PR-M-3. The structures of 1–3 were elucidated on the basis of spectral data, single-crystal X-ray diffraction, and ECD analysis. Compounds 1 and 3 showed moderate cytotoxicity against the HL-60 cell line.
Entomopathogenic fungi are considered as potential sources of bioactive compounds for their ability to produce various secondary metabolites.1,2 Lecanicillium is a genus of entomopathogenic fungi that was previously widely known as Lecanicillium (formerly Verticillium) lecanii (Zimmerman) Viegas, and at least 15 products based on Lecanicillium spp. have been, or are in the process of being commercialized as biological pesticides, against a variety of pests in numerous countries worldwide.3 However, it should be noted that entomopathogenic fungi of the genus Lecanicillium have rarely been chemically explored, only several kinds of metabolites such as indolosesquiterpenoids,4 phenopicolinic acid analogs,5 tetracyclic diterpenoid,6 pregnanes,7 and cyclic lipodepsipeptides8 isolated from the genus of Lecanicillium, have been reported to date. Recently, our investigation of the secondary metabolites on one species of this genus, Lecanicillium sp. PR-M-3, led to three novel polyketides with new carbon skeletons, lecanicillones A–C (1–3), and their biogenetic precursor, spiciferone A (4),9 obtained from the fermentation broth (Fig. 1). In this paper, we describe their structure elucidation, plausible genetic pathway, and biological activities.
Lecanicillone A (1) was obtained as colorless block crystals (EtOAc) with [α]20D +170.3 (c 1.08, MeOH). Its molecular formula was established to be C28H32O6 (13 degrees of unsaturation) on the basis of HRESIMS at m/z 465.2276 [M + H]+ (calcd 465.2272). The IR absorption bands at 1717 and 1662 cm−1 indicated the presence of carbonyl groups. The 1H NMR spectrum of 1 exhibited the signals assigned to three methyls [δH = 1.47, 1.89, 2.26 (each 3H, s)], one ethyl [δH = 1.82 (2H, dq, J = 14.4, 7.2 Hz), 0.78 (3H, t, J = 7.6 Hz)], and two methines [δH 3.41 (1H, dd, J = 7.2, 1.0 Hz), 3.56 (1H, dd, J = 7.2, 1.0 Hz)]. The 13C NMR spectrum showed the signals of six sp2 carbons, including two carbonyl carbons, and eight sp3 carbons. The NMR data of 1 were very similar to those of spiciferone A (4),9a except for the absence of the signals due to the double bond at C-5–C-6, and instead, the existence of two additional methines (δH = 3.41 and 3.56). Combined with the molecular formula, it could allow us to infer that 1 might be a dimeric 5,6-dihydrospiciferone A with a highly symmetrical skeleton. Based on HSQC and HMBC experiments, the 1H and 13C NMR signals of 1 were assigned as shown in Table 1. In the HMBC spectrum (Fig. 2), the correlations from H-5/5′ to C-4/4′, C-4a/4a′, C-5′/5, C-7/7′ and C-8a/8a′, and from H-6/6′ to C-5/5′, C-6′/6 and C-7/7′ suggested that two 5,6-dihydrospiciferone A units should be conjugated through a cyclobutane ring by either ‘head-to-head trans-fused’ pattern (a and b in Fig. 3) or ‘head-to-tail cis-fused’ pattern (c and d in Fig. 3).
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | |
| a Measured in CDCl3 at 600 MHz for 1H and 150 MHz for 13C. | ||||||
| 2/2′ | 161.0 | 161.0 | 161.5/161.6 | |||
| 3/3′ | 120.0 | 120.7 | 120.0/120.0 | |||
| 4/4′ | 176.8 | 176.7 | 177.5/177.7 | |||
| 4a/4a′ | 119.9 | 120.1 | 119.8/119.5 | |||
| 5/5′ | 42.4 | 3.56 (1H, dd, 7.2, 1.0) | 42.2 | 3.49 (1H, m) | 35.7/34.5 | 4.13 (ddd, 8.7, 7.4, 0.9)/4.00 (ddd, 8.8, 3.7, 0.9) |
| 6/6′ | 45.1 | 3.41 (1H, dd, 7.2, 1.0) | 44.4 | 3.44 (1H, m) | 47.0/51.2 | 3.05 (ddd, 8.7, 3.7, 1.1)/2.90 (ddd, 8.8, 7.7, 1.1) |
| 7/7′ | 210.0 | 210.0 | 206.9/207.1 | |||
| 8/8′ | 51.6 | 51.8 | 51.7/51.1 | |||
| 8a/8a′ | 163.4 | 162.6 | 164.1/163.6 | |||
| 9/9′ | 33.8 | 1.82 (2H, dq, 14.4, 7.2) | 27.7 | 1.87 (1H, dq, 14.0, 7.0) | 28.3/31.9 | 1.90 (dq, 14.6, 7.2)/1.75 (dq, 14.0, 7.2) |
| 2.30 (1H, dq, 14.0, 7.0) | 2.26 (dq, 14.6, 7.2)/2.05 (dq, 14.0, 7.2) | |||||
| 10/10′ | 9.3 | 0.78 (3H, t, 7.6) | 10.3 | 0.84 (3H, t, 7.2) | 10.3/9.5 | 0.90 (3H, t, 7.2)/0.61 (3H, t, 7.2) |
| 11/11′ | 9.9 | 1.89 (3H, s) | 9.8 | 1.91 (3H, s) | 9.7/9.6 | 1.94 (3H, s)/1.99 (3H, s) |
| 12/12′ | 17.8 | 2.26 (3H, s) | 17.7 | 2.29 (3H, s) | 17.8/17.8 | 2.31 (3H, s)/2.34 (3H, s) |
| 13/13′ | 18.7 | 1.47 (3H, s) | 27.0 | 1.39 (3H, s) | 26.7/22.7 | 1.35 (3H, s)/1.75 (3H, s) |
Further, the relative stereochemistry of compound 1 was determined by NOESY experiment (Fig. 4). The NOE correlations between H-5/5′ and H-6/6′ indicated that H-5 and H-6 (or/and H-5′ and H-6′) were the same orientation. Moreover, the correlations between H-5/5′ and H-13/13′ as well as between H-6/6′ and H-9/9′, H-10/10′ were also observed. Considering the highly symmetrical skeleton, compound 1 should be derived from ‘head-to-head trans-’ cyclization of 4 and possesses the two possible structures (a or b in Fig. 3), which could not be distinguished by analysis of the NMR data. Fortunately, crystal of 1 suitable for a single-crystal X-ray diffraction experiment was obtained (Fig. 5). Thus, the absolute configuration of 1 was unambiguously determined to be 5S,5′S,6S,6′S,8R,8′R (i.e. structure a in Fig. 3).
Lecanicillone B (2) was obtained as white amphorous powder with [α]20D −107.0 (c 0.15, MeOH). Its molecular formula was established to be C28H32O6 on the basis of HRESIMS at m/z 465.2257 [M + H]+ (calcd 465.2272). Its UV, IR, and NMR data (Table 1) were very similar to those of 1, implying 2 also to be a highly symmetrical 5,6-dihydrospiciferone A dimer fused through a cyclobutane ring (Fig. 2). According to the NOE correlations of H-5/5′ with H-6/6′ and H-10/10′, and H-6/6′ with H-5/5′ and H-13/13′ (Fig. 4), we inferred that 2 probably was the other ‘head-to-head’ trans-fused dimer of 4 and had the structure b as shown in Fig. 3. This inference was further confirmed by comparison of the ECD spectrum of 2 with that of 1 (Fig. 6). The ECD curve of 2 showed the Cotton effects opposite to those of 1 at almost all detected wavelength, except for the region of 230–250 nm. Since the configuration of C-8 in the precursor (spiciferone A, 4) has been determined to be R as demonstrated in compound 1 by analysis of the single-crystal X-ray diffraction, the absolute stereochemistry of 2 was assigned as 5R,5′R,6R,6′R,8R,8′R. This conclusion was also supported by the result of quantum chemical ECD calculations (Fig. 6).
Lecanicillone C (3) was obtained as white amphorous powder with [α]20D +28 (c 0.11, MeOH). The molecular formula C28H32O6 based on the HRESIMS at m/z 465.2262 [M + H]+ (calcd 465.2272) suggested that 3 was another dimer of 4 with a cyclobutane ring. However, the 1H and 13C NMR spectra of 3 (Table 1) exhibited two separated sets of signals for the two 5,6-dihydrospiciferone A units, indicating that it was an unsymmetrical dimer. According to the HMBC correlations of H-5 with C-4a, C-6, C-7, C-8a and C-6′, and H-6′ with C-5, C-6, C-4a′, C-7′, as well the 1H–1H COSY correlations from H-5/5′ to H-6 and H-6′, H-6/6′ to H-5 and H-5′, the planar structure of 3 derived from the ‘head-to-tail’ fused dimer was deduced as shown in Fig. 2. Moreover, the NOESY correlations of H-5 with H-6 and H-13′, and H-6 with H-5 and H-13 revealed that H-5, H-6, H-13, and H-13′ were on the same side of the ring system. Meanwhile, the NOESY correlations between H-5′ and H-6′, H-10′, H-10, and between H-6′ with H-5, H-10′, H-10 showed that H-5′ and H-6′, H-10′, and H-10 located on the other side (Fig. 4).
In the ECD spectrum of 3, the Cotton effect closed to zero at 260–280 nm instead of the remarkable peaks in those of 1 and 2 also implied that the configurations of C-5 and C-5′, as well as C-6 and C-6′ in the two monomers might be opposite, respectively. Similar to 2, the absolute stereochemistry of 3 was finally identified as 5S,5′R,6R,6′S,8R,8′R by comparison of the calculated ECD spectra with the experimental one (Fig. 7).
Spiciferones are a rare group of fungal metabolites containing a bicyclic unit composed of a fully substituted α-pyrone and a cyclohexadienone. To our best knowledge, no more than 20 spiciferones and the biogenetically related compounds have been isolated from the fungi Cochliobolus spicifer,9 Drechslera hawaiiensis,10 Penicillium sp.11 and Pestalotiopsis disseminate12 to date, and this is the first report for the dimeric spiciferones with cyclobutane ring.
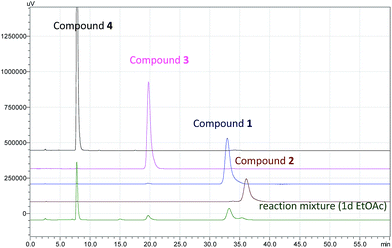
The biosynthesis of spiciferone A (4) derived from a hexaketide and two C1 units, after undergoing modifications including the unique C–C bond cleavage by retroaldol condensation.13 Compounds 1–3 were formed by [2 + 2] cycloaddition from the precursor 4. Although [2 + 2] cycloaddition is generally considered as a photoreaction14 and 1–3 had been really detected from the mixture of the photochemical reaction using 4 as a reactant (Fig. 8), only 1 was also observed with high content in the EtOAc crude extract of the fermentation broth cultured in dark (Fig. S37†). It is suggested that 1 maybe formed by both enzyme and photoreaction, while 2 and 3 were probably produced by light-irradiation in the process of extraction and isolation. Anyway, compound 1 was the major product because it is steric advantage when both of two monomers (4) were added to each other from the same side of the methyl at C-8 rather than from that of the ethyl at C-8 in the case of 2 or 3. However, more systematic research is necessary to elucidate that compound 1 is a biosynthetic (enzymatic) product.
The cytotoxic activities of compounds 1–4 were tested against three human cancer cell lines, including human leukemia HL-60, human colon cancer HCT-116, and human pancreatic cancer ASPC1, using 5-fluorouracil as positive control (ESI†). The dimers 1 and 3 showed moderate growth inhibitory activities toward HL-60 cell line with IC50 values of 47.8 and 53.0 μM, respectively, and the precursor 4 showed cytotoxic activities against HCT-116 and ASPC1 cell lines with IC50 values of 31.8 and 32.3 μM, respectively (Table S1†). The primary insecticidal activities of compound 1 against four kinds of insects were also evaluated (ESI†). As a result, 1 showed no insecticidal activity at the concentration of 600 mg L−1.
Acknowledgements
This work was supported by the General Research Project of Educational Commission of Liaoning Province of China (L2014376). It was also supported by Program for Innovative Research Team of the Ministry of Education and Program for Liaoning Innovative Research Team in University. We gratefully acknowledge Prof. Jian Hao, Department of Analytical Testing Center, Beijing University of Chemical Technology, for the test of the X-ray diffraction.Notes and references
- M. Isaka, P. Kittakoop, K. Kirtikara, N. L. Hywel-Jones and Y. Thebtaranonth, Acc. Chem. Res., 2005, 38, 813–823 CrossRef CAS PubMed.
- I. Molnar, D. M. Gibson and S. B. Krasnoff, Nat. Prod. Rep., 2010, 27, 1241–1275 RSC.
- M. R. d. Faria and S. P. Wraight, Biol. Control, 2007, 43, 237–256 CrossRef.
- D. M. Roll, L. R. Barbieri, R. Bigelis, L. A. McDonald, D. A. Arias, L.-P. Chang, M. P. Singh, S. W. Luckman, T. J. Berrodin and M. R. Yudt, J. Nat. Prod., 2009, 72, 1944–1948 CrossRef CAS PubMed.
- A. G. Soman, J. B. Gloer, R. F. Angawi, D. T. Wicklow and P. F. Dowd, J. Nat. Prod., 2001, 64, 189–192 CrossRef CAS.
- N. Claydon and J. F. Grove, J. Invertebr. Pathol., 1982, 40, 413–418 CrossRef CAS.
- (a) N. Claydon, J. F. Grove, M. Pople and M. J. Begley, J. Chem. Soc., Perkin Trans. 1, 1984, 497–502 RSC; (b) J. F. Grove, Phytochemistry, 1984, 23, 1721–1723 CrossRef CAS.
- K. Ishidoh, H. Kinoshita, Y. Igarashi, F. Ihara and T. Nihira, J. Antibiot., 2014, 67, 459–463 CrossRef CAS PubMed.
- (a) H. Nakajima, T. Hamasaki and Y. Kimura, Agric. Biol. Chem., 1989, 53, 2297–2299 CAS; (b) H. Nakajima, T. Hamasaki, S. Maeta, Y. Kimura and Y. Takeuchi, Phytochemistry, 1990, 29, 1739–1743 CrossRef CAS; (c) H. Nakajima, T. Hamasaki, M.-A. Kohna and Y. Kimura, Phytochemistry, 1991, 30, 2563–2565 CrossRef CAS; (d) H. Nakajima, Y. Kimura and T. Hamasaki, Phytochemistry, 1992, 31, 105–107 CrossRef CAS.
- R. A. Edrada, V. Wray, A. Berg, U. Gräfe, Sudarsono, G. Brauers and P. Proksch, Z. Naturforsch., C: J. Biosci., 1999, 55, 218–221 Search PubMed.
- A. A. Stierle, D. B. Stierle and K. Kelly, J. Org. Chem., 2006, 71, 5357–5360 CrossRef CAS PubMed.
- I. H. Hwang, D. C. Swenson, J. B. Gloer and D. T. Wicklow, J. Nat. Prod., 2016, 79, 523–530 CrossRef CAS PubMed.
- (a) H. Nakajima, R. Matsumoto, Y. Kimura and T. Hamasaki, J. Chem. Soc., Chem. Commun., 1992, 1654–1656 RSC; (b) H. Nakajima, H. Fujimoto, R. Matsumoto and T. Hamasaki, J. Org. Chem., 1993, 58, 4526–4528 CrossRef CAS.
- (a) X.-J. Zhang, L.-Y. Li, S.-S. Wang, S. Que, W.-Z. Yang, F.-Y. Zhang, N.-B. Gong, W. Cheng, H. Liang, M. Ye, Y.-X. Jia and Q.-Y. Zhang, Tetrahedron, 2013, 69, 11074–11079 CrossRef CAS; (b) B.-X. Zhao, Y. Wang, C. Li, G.-C. Wang, X.-J. Huang, C.-L. Fan, Q.-M. Li, H.-J. Zhu, W.-M. Chen and W.-C. Ye, Tetrahedron Lett., 2013, 54, 4708–4711 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1037052. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra11422a |
| This journal is © The Royal Society of Chemistry 2016 |








![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: