The investigation of non-noble metal doped mesoporous cobalt oxide catalysts for the water–gas shift reaction†
Hyun-Suk Naa,
Chang-Il Ahnb,
Ajay Jhaa,
Kyung Soo Parkb,
Won-Jun Janga,
Jae-Oh Shima,
Dae-Woon Jeongc,
Hyun-Seog Roh*a and
Jong Wook Bae*b
aDepartment of Environmental Engineering, Yonsei University, 1 Yonseidae-gil, Wonju, Gangwon 220-710, South Korea. E-mail: hsroh@yonsei.ac.kr; Fax: +82-33-760-2571
bSchool of Chemical Engineering, Sungkyunkwan University (SKKU), Suwon, Geonggi-do 440-746, South Korea. E-mail: finejw@skku.edu; Fax: +82-31-290-7272
cDepartment of Environmental Engineering, Changwon National University, Changwong, Gyeongnam 641-773, South Korea
First published on 25th May 2016
Abstract
In this study, we report an investigation of the low temperature water–gas shift (LT-WGS) reaction over a series of non-noble metal doped (Me = Mn, Fe, Co, and Ni) mesoporous Co3O4 catalysts. The effect of metal dopants on the structure and reducibility of the mesoporous Co3O4 oxide was examined using X-ray diffraction (XRD), N2-adsorption/desorption isotherm measurements, and H2-temperature programmed reduction (TPR) measurements. Experimental results revealed that among the Me-doped Co3O4 catalysts, Ni/Co3O4 demonstrated the highest catalytic performance (XCO = 93% with 47% H2 yield at 280 °C). The higher activity of the Ni-doped Co3O4 catalyst was mainly due to its smaller crystallite size (8.6 nm) and strong interaction between Co and Ni, which lead to the higher reducibility of Co3O4 compared to the other metal-doped Co3O4. To further optimize the loading of Ni- over the mesoporous Co3O4, a series of Ni(x%)/Co3O4 catalysts were prepared by varying the Ni-loading in the range of 3 to 15 wt%. Among these catalysts, 5 wt% Ni- was found to be the optimum loading, whereas higher Ni-loaded samples (10 and 15 wt%) showed a decrease in catalytic performance and hydrogen yield during the WGS reaction.
Introduction
The water–gas shift (WGS) reaction plays a crucial role in low temperature fuel cell technology for on-board purification of hydrogen sources.1 Industries heavily depend on hydrocarbon reforming for the production of hydrogen, which produces a mixture of H2 and CO as the main products.2,3 The presence of insignificant amounts of CO in the hydrogen stream can deactivate the fuel-cell electrodes because the CO tolerance level of the Pt-electrode is below 30 ppm.4 To overcome this problem, the reformed gas is passed through several fuel processing steps, including CO removal by means of the WGS and CO preferential oxidation (PrOx). The conventionally used low temperature (LT)-WGS catalyst composed of Cu–Zn–Al ternary oxides, converts CO into CO2 and produces hydrogen in the temperature range of 250–400 °C.5–7 Although the conventional catalyst (Cu–Zn–Al) exhibits a high WGS activity, it is pyrophoric and normally requires lengthy and complex activation steps before usage.8,9Recently, our group focused on the development of mesoporous catalysts for use in the WGS reaction.10,11 Our previous studies demonstrated that mesoporous material provides several advantages in the WGS reaction: (1) the ordered porous structures ensure an uninterrupted diffusion of reactant and product molecules; (2) well-dispersed metal oxide over the mesoporous support provides more accessible active sites, and (3) a higher catalyst surface area is achieved than the metal oxide supported on a non-mesoporous oxide. Here, we report the successful fabrication of Mn-, Fe-, Cu- and Ni-doped mesoporous Co3O4 catalysts and evaluated their catalytic performance in the LT-WGS reaction. The transition metals Mn, Fe, Cu, Ni and Co were selected because of their low cost and strong redox properties. The WGS reaction is catalyzed by associated or redox mechanism, therefore a highly reducible metal oxide catalyst can facilitate the WGS reaction. Ordered mesoporous Co3O4 has been widely used in catalysis and has displayed excellent stability under harsh reaction conditions.12 Co3O4 is a reducible oxide that has received considerable attention in catalysis due to the weaker Co–O bond strength and low hopping barrier of oxygen vacancies on the surface compared to the other 3d transition metal series.1 The presence of oxygen vacancies promotes lattice-oxygen mobility in Co3O4, creating its interest as an active catalyst for CO oxidation, PrOx and WGS reactions.13–15 The nanocasting route using silica materials as a hard template was used for the synthesis of ordered mesoporous Co3O4.16 The as prepared mesoporous Co3O4 materials showed a large surface area (∼89 m2 g−1) and a uniform pore size distribution (ESI Fig. S1†), which made it ideal for use as a support. As prepared Me-doped mesoporous Co3O4 catalysts were used in the LT-WGS reaction. The results showed that in the presence of dopants, the reducibility of Co3O4 has increased, which in turn affected the CO conversion and H2 yield. Among the Me-doped mesoporous Co3O4 catalysts, Ni-doped mesoporous Co3O4 exhibited excellent catalytic performance. Further, to optimize the loading of Ni- over the mesoporous Co3O4, a series of Ni(x%)/Co3O4 catalysts were prepared by varying the Ni-loading in the range of 3 to 15 wt%. The effect of Ni-loadings on the mesoporous structure of Co3O4 was examined using N2 adsorption/desorption isotherms, and those results have been correlated to the catalytic performance in the LT-WGS reaction.
Results and discussion
Effects of Mn-, Cu-, Fe-, and Ni-doped Co3O4
The composition and phase purity of Mn-, Fe-, Ni-, and Cu-doped mesoporous Co3O4 were examined by XRD and the results are shown in Fig. 1(A). Well-defined diffraction peaks indicate the crystalline nature of the samples, which can be indexed well to the face-centered cubic phase of Co3O4 spinel.17 No diffraction peaks related to dopants were observed in any of the Me-doped Co3O4 samples. This might be caused by the uniform dispersion of dopant over the mesoporous Co3O4 or the formation of a solid solution of dopant and Co3O4. The crystallite sizes of the Me-doped Co3O4 samples were estimated using the Debye–Scherrer equation for the full-width at half-maximum (FWHM) of the (311) reflection at 2θ = 36.7°. The results are summarized in Table 1. Mn-, Fe- and Cu-doped mesoporous Co3O4 samples show higher crystallite size, however Ni-doped sample exhibits decreased crystallite size compared to the pure mesoporous Co3O4. The reduction in crystallite size of mesoporous Co3O4 upon doping with Ni- could be ascribed to the loss of crystallinity or deformation in the hexagonal structure of Co3O4.| Catalyst | Surface area (m2 g−1) | Crystallite size of Co3O4 (nm) |
|---|---|---|
| Co3O4 | 89 | 11 |
| Mn/Co3O4 | 52 | 14 |
| Cu/Co3O4 | 49 | 13 |
| Fe/Co3O4 | 65 | 13 |
| Ni/Co3O4 | 36 | 8.6 |
H2-TPR experiments were used to investigate the doping effect of Mn-, Fe-, Ni-, and Cu- on the reducibility of mesoporous Co3O4. As shown in Fig. 1(B), pure mesoporous Co3O4 exhibits two broad reduction peaks. The first peak centered at 292 °C is associated with the reduction of trivalent cobalt oxide (Co3O4) to divalent cobalt oxide (CoO) (eqn (1)), and the second peak centered at 415 °C is associated with the subsequent reduction of divalent cobalt oxide (CoO) to metallic cobalt (Coo) (eqn (2)).18
| Co3O4 + H2 → 3CoO + H2O | (1) |
| 3CoO + 3H2 → 3Coo + 3H2O | (2) |
All the Me-doped mesoporous Co3O4 catalysts show broad TPR profiles, which implies a multistage reduction of the catalyst. Mn/Co3O4 shows three reduction peaks around 338, 419 and 497 °C, corresponding to a reduction of Mn3O4, Co3O4 and CoO, respectively.19 The significant increase in the Co3O4 and CoO reduction temperature in the case of Mn/Co3O4 may be attributed to the formation of spinel CoMn2O4. Cu/Co3O4 catalyst shows three reduction peaks around 211, 286 and 337 °C, corresponding to the reduction of highly dispersed CuO, Co3O4 and CoO, respectively.11 The H2-TPR profile of Fe/Co3O4 exhibits three peaks around 331, 356, and 515 °C, which are likely related to the reductions of Fe2O3, Co3O4, and CoO, respectively.20 Ni/Co3O4 displays two reduction peaks around 285 and 405 °C, which are attributed to the reduction of Co3O4, and a combined reduction peak due to the NiO and CoO, respectively.21 The reduction temperature of tri-cobalt oxide (Co3O4) in the prepared catalysts follows the order: Mn/Co3O4 (419 °C) > Fe/Co3O4 (356 °C) > Co3O4 (292 °C) > Cu/Co3O4 (286 °C) > Ni/Co3O4 (285 °C). These results imply that the presence of a second metal in the mesoporous Co3O4 framework lowered the reduction temperature for Co3O4 except for Fe/Co3O4 and Mn/Co3O4 catalysts. This might be due to the formation of CoFe2O4 and CoMn2O4. The lower reduction temperatures of tri-cobalt oxide in the case of Cu- and Ni-doped Co3O4 might be due to the strong interaction between Co and the doped metal. Among the Me-doped Co3O4 samples, Ni/Co3O4 shows the lowest temperature for Co3O4 reduction, which can be attributed to the weak Co–O bond strength caused by the strong interaction between Co and Ni. The decrease in Co–O bond strength in Co3O4 on doping of Ni- can lead to enhanced oxygen vacancies on the catalyst surface. These oxygen vacancies are considered as active sites for the water dissociation in WGS reaction.11
Catalyst performance in the LT-WGS reaction
The catalytic performances of Me-doped Co3O4 catalysts were investigated in the LT-WGS reaction. The CO conversion over Me-doped Co3O4 catalysts as a function of reaction temperature is shown in Fig. 2(A). The results showed that Ni/Co3O4 possess the highest catalytic activity (>93% CO conversion at 280 °C) compared to the Mn-, Fe- and Cu-doped Co3O4 catalysts (<50% CO conversion at 280 °C). However, Mn/Co3O4 exhibited inferior activity compared to the support (mesoporous Co3O4) itself. This implies that the doping of mesoporous Co3O4 with Mn- caused a negative impact on the CO conversion. Interestingly, at 280 °C, the activity patterns of Me-doped mesoporous Co3O4 catalysts follow the same trend as the reducibility of Co3O4 in Me-doped mesoporous Co3O4: Ni/Co3O4 > Cu/Co3O4 > Co3O4 > Fe/Co3O4 > Mn/Co3O4. This result suggests that the reducibility of Co3O4 is crucial to attaining the high activity in the LT-WGS reaction. Fig. 2(B) depicts the hydrogen yields in the LT-WGS reaction for all the Me-doped mesoporous Co3O4 catalysts as a function of reaction temperature. The yield of hydrogen was negligible at 240 °C for Mn-, Fe- and Cu-doped mesoporous Co3O4 catalysts. However, at the same temperature, Ni/Co3O4 exhibited a 7% hydrogen yield and it increased with further increases of the reaction temperature. Fig. 2(B) reveals that the Ni/Co3O4 catalyst has the highest hydrogen yield throughout the entire temperature range among the Me-doped Co3O4 catalysts in LT-WGS. | ||
Fig. 2 CO conversion (A) and hydrogen yield (B) as a function of reaction temperature over Ni/Co3O4 catalysts with varying Ni loading (H2O/(CH4 + CO + CO2) = 2.0; GHSV = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 h−1). 027 h−1). | ||
Effect of Ni-loadings on the mesoporous Co3O4
Among the various catalysts screened for the LT-WGS reaction, Ni/Co3O4 exhibited the best catalytic performance. To investigate the optimum loading of Ni on the mesoporous Co3O4 in order to achieve both the highest CO conversion and H2 yield, we prepared a series of Ni(x%)/Co3O4 catalysts (x = wt% of Ni) by varying the Ni-loading in the range of 3 to 15 wt%. The detailed characterization and activity results of these catalysts are discussed in the subsequent sections. Fig. 3(A) displays the XRD patterns of Ni(x%)/Co3O4 catalysts with various Ni-loadings. The peak positions and intensity of Co3O4 show no significant change upon doping with up to 10 wt% Ni-, however, the 15 wt% Ni-loaded samples show a slightly increased in the intensity of the (311) peak. The crystallite size of Ni(x%)/Co3O4 catalysts was estimated from (311) diffraction peak using the Scherrer formula and these results are summarized in Table 2. As shown, the crystallite size of mesoporous Co3O4 decreases from 11 to 8.2 nm after loading with 3 wt% Ni. A similar size is maintained up to the 10 wt% Ni-loading. However, a further increase in the Ni-loading to 15 wt% resulted in a crystallite size increase to the same value (11 nm) as that observed for pure mesoporous Co3O4. This suggests that excess loadings of Ni – on the mesoporous Co3O4 led to a decrease in Ni–Co interactions, which do not affect the crystallite size of the mesoporous cobalt. In addition, no distinct XRD peaks correspond to the metal or oxide phase of Ni- are observed up to a 10 wt% Ni-loading. This implies that the dopant (Ni-) was uniformly dispersed over the Co3O4 matrix up to 10 wt% loading. However, above this loading, a NiO peak starts to appear in the case of Ni(15%)/Co3O4 catalyst.| Catalyst | Surface area (m2 g−1) | Crystallite size of Co3O4 (nm) | Pore volume (cm3 g−1) | H2 consumption at 330–516 °C (cm3 g−1) |
|---|---|---|---|---|
| Co3O4 | 89 | 11 | 0.18 | 205 |
| Ni(3%)/Co3O4 | 74 | 8.2 | 0.17 | 207 |
| Ni(5%)/Co3O4 | 36 | 8.6 | 0.15 | 210 |
| Ni(10%)/Co3O4 | 29 | 8.7 | 0.11 | 216 |
| Ni(15%)/Co3O4 | 45 | 11 | 0.16 | 220 |
However, above this loading, a NiO peak starts to appear in the case of Ni(15%)/Co3O4 catalyst. This might be caused by the segregation of the NiO from the support at higher Ni-loadings.
In order to investigate the effect of Ni-loading on the reducibility of Co3O4, TPR measurement was carried out using H2. H2-TPR profiles of Ni(x%)/Co3O4 catalysts are presented in Fig. 3(B). All the samples exhibit broad reduction peak profiles, which could be due to the overlapping contribution from different components in Ni(x%)/Co3O4. In general, two main reduction peaks can be discerned. The first reduction peak in the temperature range of 200–300 °C corresponded to the reduction of Co3O4 to CoO. The second peak at about 330–516 °C is attributed to the combined reduction peaks of NiO and CoO. The amount of H2 consumed in this temperature range (330–516 °C) is measured and summarized in Table 2.
As shown, the amount of hydrogen consumed increases with increase of Ni-loading. However, with the increase of the Ni-loading from 5 to 10% and then 15%, the H2 consumption amount does not increase to double or triple as expected. This might be due to the poor dispersion of Ni over the mesoporous Co3O4 on excess loadings of Ni. The reduction temperature of tri-cobalt oxide in Ni(3%)/Co3O4 is the same as that for the pure mesoporous Co3O4. However, Ni(x%)/Co3O4 catalysts (x = 5 to 15%) show a lower temperature (∼285 °C) for reduction of tri-cobalt oxide compared to pure mesoporous Co3O4. N2-adsorption/desorption measurement was carried out to investigate the effect of Ni-loadings on the textural property of mesoporous Co3O4. ESI Fig. S2† displays the isotherm plots of pure mesoporous Co3O4 and Ni(x%)/Co3O4 catalysts. The pure mesoporous Co3O4, Ni(3%)/Co3O4 and Ni(5%)/Co3O4 exhibit a type IV isotherm with a H1-type hysteresis loop, which is characteristic of mesoporous materials. However, Ni(10%)/Co3O4 and Ni(15%)/Co3O4 catalysts showed a type III isotherm, which corresponds to the non-mesoporous materials. The formation of non-mesoporous materials is caused by the deposition of excess Ni- over the mesoporous framework of Co3O4 that results in pore blockage. This observation was supported by the pore volume as shown in Table 2.
Catalytic performance over Ni(x%)/Co3O4
The catalytic performance of the Ni(x%)/Co3O4 catalysts in the LT-WGS reaction is shown in Fig. 4. Notably, the un-doped mesoporous Co3O4 was moderately active in the LT-WGS reaction. However, doping of Ni- over the mesoporous Co3O4 gradually enhanced the catalytic efficiency of Ni(x%)/Co3O4 catalysts. The catalytic activity increased with increasing the loading amount of Ni- from 3 to 5 wt% and reached a maximum at 5 wt% of Ni-loading (XCO = 93% at 280 °C). Further increase in the Ni-loading (10 and 15 wt%) resulted in a lower catalytic performance compared to the Ni(5%)/Co3O4 catalyst. The highest CO conversion of Ni(5%)/Co3O4 could be attributed to the mesoporous structure of the catalyst which was absent in Ni(10%)/Co3O4 and Ni(15%)/Co3O4 catalysts. Therefore, 5 wt% Ni supported on mesoporous Co3O4 can be considered as the optimum loading as it provided the high activity in the LT-WGS reaction. Fig. 4(B) shows the H2 yields during the WGS reaction over the Ni(x%)/Co3O4 catalysts as a function of reaction temperature. It revealed that the yields of hydrogen increased after doping of Ni over the mesoporous Co3O4 except for Ni(15%)/Co3O4. Table S1† shows a comparison of reported data on CO conversion for the WGS reaction using Co based catalysts. Clearly, catalytic activity of Ni/Co3O4 catalyst shows higher activity in WGS reaction.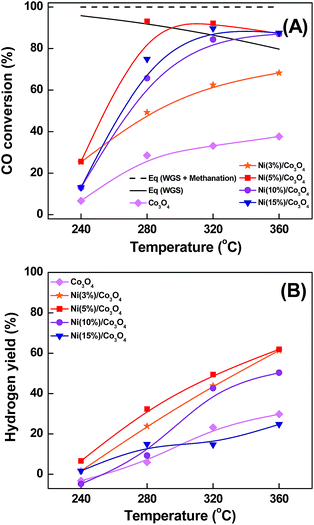 | ||
Fig. 4 (A) CO conversion and (B) hydrogen yield as a function of reaction temperature over Ni(x%)/Co3O4 catalysts with varying Ni loading (H2O/(CH4 + CO + CO2) = 2.0; GHSV = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 h−1). 027 h−1). | ||
Among the Ni(x%)/Co3O4 series, the Ni(5%)/Co3O4 catalyst showed the highest yield of hydrogen throughout the temperature range (240 to 360 °C) compared to other Ni(x%)/Co3O4 catalysts. The Ni(10%)/Co3O4 and Ni(15%)/Co3O4 catalysts showed a reduction in hydrogen yield. This can be attributed to the formation of CH4, as the bulk Ni species are the active sites for methane formation (CO + 3H2 → CH4 + H2O) under WGS reaction conditions. As the formation of one mole of methane consumed 3 moles of H2, the production of methane can decrease the H2 yield in the output gas of WGS reaction.
To check the potential of the Ni(5%)/Co3O4 catalyst, a time-on-stream study was performed at 320 °C and at the GHSV of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 201 h−1. Fig. 5 shows the CO conversion as a function of time. These results demonstrate that the CO conversion decreased during the initial period of 10 h from 92 to 86%, thereafter, the catalyst maintained a stable activity up to 50 h time-on-stream. The morphology of the fresh and used Ni(5%)/Co3O4 catalyst was investigated by TEM, as shown in Fig. 6. The average particle sizes of the fresh and used Ni(5%)/Co3O4 catalyst were calculated to be 12 and 18 nm, respectively. This observation implies that the Ni(5%)/Co3O4 catalyst undergoes gradual particle growth during the time-on-stream study caused by catalyst sintering.
201 h−1. Fig. 5 shows the CO conversion as a function of time. These results demonstrate that the CO conversion decreased during the initial period of 10 h from 92 to 86%, thereafter, the catalyst maintained a stable activity up to 50 h time-on-stream. The morphology of the fresh and used Ni(5%)/Co3O4 catalyst was investigated by TEM, as shown in Fig. 6. The average particle sizes of the fresh and used Ni(5%)/Co3O4 catalyst were calculated to be 12 and 18 nm, respectively. This observation implies that the Ni(5%)/Co3O4 catalyst undergoes gradual particle growth during the time-on-stream study caused by catalyst sintering.
 | ||
Fig. 5 CO conversion with time-on-stream over Ni(5%)/Co3O4 catalyst (H2O/(CH4 + CO + CO2) = 2.0; T = 320 °C; GHSV = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 h−1). 027 h−1). | ||
 | ||
| Fig. 6 TEM images of pure mesoporous Co3O4 (A), fresh Ni(5%)/Co3O4 (B) and used Ni(5%)/Co3O4 (C) catalysts. | ||
Conclusions
We have successfully synthesized Mn-, Fe-, Ni- and Cu-doped mesoporous Co3O4 catalysts and evaluated their catalytic efficiency in the LT-WGS reaction. Among these metal-doped mesoporous Co3O4 catalysts, Ni/Co3O4 exhibited the highest CO conversion and H2 yield compared to the Mn/Co3O4, Fe/Co3O4 and Cu/Co3O4 catalysts. The higher activity of Ni/Co3O4 is attributed to the strong interaction between Co and Ni, which led to the easier reducibility of mesoporous Co3O4 support. Furthermore, loading of Ni- over the mesoporous Co3O4 was optimized for the improved performance in the LT-WGS reaction. The results showed that 5 wt% loading of Ni was optimum for the catalyst performance in the LT-WGS reaction. The time-on-stream study showed that Ni(5%)/Co3O4 catalyst maintained stable activity performance for 50 h with 85% CO conversion. This finding suggests that Ni(5%)/Co3O4 catalyst can be regarded as a potential catalyst in the LT-WGS reaction.Experimental
Catalyst preparation
Mesoporous Co3O4 was synthesized through a nano-casting method using KIT-6 followed by a subsequent incipient wetness impregnation method of cobalt precursor in similar to that of previously reported works.22 The ordered mesoporous KIT-6, which has a three-dimensional hexagonal regular arrangement of uniform mesopores, was synthesized using an amphiphilic co-polymer according to the corresponding literature.23 A triblock copolymer, EO20PO70EO20 (Pluronic P123, Aldrich), was used as the structure-directing agent, and tetraethylorthosilicate (TEOS, Aldrich), was used as the silica source. P123 was dissolved completely in deionized water. A polymer solution was added to a 35 wt% HCl aqueous solution with vigorous stirring at 35 °C. Subsequently, n-butanol was added and aged for 1 h with stirring. Then, TEOS was added to the solution under stirring in order to cover the hexagonal array of micelle rods with silica. The final mixed solution was vigorously stirred for 24 h at 35 °C. The product of milky solution was transferred to an autoclave and kept for 24 h at 100 °C under static conditions. The hydrothermally-treated white solid product was filtered without washing, and subsequently dried at 100 °C for 24 h. To remove the surfactant, an ethanol/HCl solution was used with stirring for about 2 h, and the as-prepared KIT-6 powder was calcined at 550 °C for 6 h. In order to prepare mesoporous Co3O4, cobalt nitrate hexahydrate precursor was dissolved in deionized water, and the solution was impregnated into the pores of KIT-6 by homogenous mixing. The well-mixed gel of the cobalt precursor solution with KIT-6 powder was dried in an oven at 80 °C overnight and calcined at 550 °C for 3 h. Finally, KIT-6, used as a template of mesoporous Co3O4, was removed by washing with a 2 M NaOH solution to obtain template-free mesoporous Co3O4 metal oxides.For preparing mesoporous Me/Co3O4 (Me = Mn, Fe, Cu, and Ni) catalysts, the corresponding Me-precursors were added through an impregnation method with a metal loading of 5 wt% on the mesoporous Co3O4. The required amount of Me-precursors was dissolved in deionized water and mixed with the Co3O4. Then, Me/Co3O4 samples were dried at 110 °C for 6 h, and calcined at 400 °C for 6 h. The Ni(x%)/Co3O4 catalyst series were also synthesized using the same preparation method by varying the Ni-loadings from 3 to 15 wt%. Where, x represents the Ni-loading on the mesoporous Co3O4 oxide.
Catalyst characterization
X-ray diffractograms of the catalysts were recorded in the 2θ range of 20–80° using a Rigaku D/MAX-IIIC diffractometer (Ni-filtered Cu-Kα radiation, 40 kV, 100 mA). The crystallite size was estimated using the Debye–Scherrer equation.24,25 The values of the full width at half maximum (FWHM) were obtained for the peak of Co3O4 at 2θ = 36.8°. The BET surface area and the type of isotherm were determined by the N2 adsorption/desorption method at 77 K using an ASAP 2010 Micromeritics. The pore size distributions of Co3O4 catalysts were also calculated using the BJH (Barrett–Joyner–Halenda) model from the desorption branch of the nitrogen isotherm. Hydrogen-temperature programmed reduction (H2-TPR) experiments were conducted on an Autochem 2920 (Micromeritics). Typically, 0.1 g of sample was loaded into a quartz reactor. The H2-TPR was performed using 10% H2 in Ar with a heating rate of 10 °C min−1, from room temperature to 800 °C. The sensitivity of the detector was calibrated by reducing a known weight of NiO. A detailed procedure for the H2-TPR measurement was provided in a previous study.26,27 Surface morphologies of mesoporous Co3O4 catalyst and used catalysts, which were previously washed with hexane solvent to remove adsorbed soluble wax components on the Co3O4 surfaces, were also characterized by transmission electron microscopy (TEM) using a TECNAI G2 instrument operated at 200 kV.Catalytic activity measurement
The catalytic activities of the metal doped mesoporous cobalt oxide samples were evaluated in a fixed-bed micro-tubular quartz reactor under atmospheric pressure from 240 to 360 °C. The catalyst charge was 48 mg and reduced in 5% H2/N2 at 400 °C for 1 h prior to catalytic measurements. After pretreatment, the temperature was decreased to 200 °C. The simulated reformed gas consisted of 6.5 vol% CO, 7.1 vol% CO2, 0.7 vol% CH4, 42.4 vol% H2, 28.7 vol% H2O, and 14.5 vol% N2. The feed H2O/(CH4 + CO + CO2) ratio was intentionally fixed at 2.0 to avoid coke formation.28–30 A GHSV of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 h−1 was used to screen the catalysts in this study. Water was fed using a syringe pump and was vaporized at 180 °C upstream of the reactor. The reformed gas was chilled, passed through a trap to condense the residual water, and then analyzed on-line using a micro-gas chromatograph (Agilent 3000).
027 h−1 was used to screen the catalysts in this study. Water was fed using a syringe pump and was vaporized at 180 °C upstream of the reactor. The reformed gas was chilled, passed through a trap to condense the residual water, and then analyzed on-line using a micro-gas chromatograph (Agilent 3000).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2013R1A1A1A05007370).References
- S. Zhang, J.-J. Shan, Y. Zhu, A. I. Frenkel, A. Patlolla, W. Huang, S. J. Yoon, L. Wang, H. Yoshida, S. Takeda and F. Tao, J. Am. Chem. Soc., 2013, 135, 8283 CrossRef CAS PubMed.
- V. Subramanian, D.-W. Jeong, W.-B. Han, W.-J. Jang, J.-O. Shim and H.-S. Roh, Int. J. Hydrogen Energy, 2013, 38, 8699 CrossRef CAS.
- D.-W. Jeong, W.-J. Jang, H.-S. Na, J.-O. Shim, A. Jha and H.-S. Roh, J. Ind. Eng. Chem., 2015, 27, 35 CrossRef CAS.
- R. Jain and R. Maric, Appl. Catal., A, 2014, 475, 461 CrossRef CAS.
- D.-W. Jeong, W.-J. Jang, J.-O. Shim, W.-B. Han, H.-S. Roh, U. H. Jung and W. L. Yoon, Renewable Energy, 2014, 65, 102 CrossRef CAS.
- R. B. Mane, D.-W. Jeong, A. V. Malawadkar, H.-S. Roh and C. V. Rode, ChemCatChem, 2014, 6, 1698 CrossRef CAS.
- H.-S. Na, D.-W. Jeong, W.-J. Jang, J.-O. Shim and H.-S. Roh, Int. J. Hydrogen Energy, 2015, 40, 12268 CrossRef CAS.
- D.-W. Jeong, H.-S. Na, J.-O. Shim, W.-J. Jang, H.-S. Roh, U. H. Jung and W. L. Yoon, Int. J. Hydrogen Energy, 2014, 39, 9135 CrossRef CAS.
- S. C. Ammal and A. Heyden, J. Catal., 2013, 306, 78 CrossRef CAS.
- V. Subramanian, E. S. Gnanakumar, D.-W. Jeong, W.-B. Han, C. S. Gopinath and H.-S. Roh, Chem. Commun., 2013, 49, 11257 RSC.
- A. Jha, D.-W. Jeong, W.-J. Jang, C. V. Rode and H.-S. Roh, RSC Adv., 2015, 5, 1430 RSC.
- X. Wanga, W. Wen, J. Mi, X. Li and R. Wang, Appl. Catal., B, 2015, 176–177 Search PubMed.
- X. Xie, Y. Li, Z. Q. Liu, M. Haruta and W. Shen, Nature, 2009, 458, 746 CrossRef CAS PubMed.
- Y.-W. Chen, H.-J. Chen and D.-S. Lee, J. Mol. Catal. A: Chem., 2012, 363–364 Search PubMed.
- K. Chayakul, T. Srithanratana and S. Hengrasmee, J. Mol. Catal. A: Chem., 2011, 340, 39 CrossRef CAS.
- D. Gu and F. Schüth, Chem. Soc. Rev., 2014, 43, 313 RSC.
- I. I. Soykal, H. Sohn and U. S. Ozkan, ACS Catal., 2012, 2, 2335 CrossRef CAS.
- A. Jha and C. V. Rode, New J. Chem., 2013, 37, 2669 RSC.
- S. Todorova, H. Kolev, J. P. Holgado, G. Kadinov, C. Bonev, R. Pereñíguez and A. Caballero, Appl. Catal., B, 2010, 94, 46 CrossRef CAS.
- R. Amrousse, A. Tsutsumi, A. Bachar and D. Lahcene, Appl. Catal., A, 2013, 450, 253 CrossRef CAS.
- D. G. Klissurski and E. L. Uzunova, Chem. Mater., 1991, 3, 1060 CrossRef CAS.
- C. Ahn, H. M. Koo, M. Jin, J. M. Kim, T. Kim, Y.-W. Suh, K. J. Yoon and J. W. Bae, Microporous Mesoporous Mater., 2014, 188, 196 CrossRef CAS.
- W. Yue and W. Zhou, Chem. Mater., 2007, 19, 2359 CrossRef CAS.
- W.-J. Jang, D.-W. Jeong, J.-O. Shim, H.-S. Roh, I. H. Son and S. J. Lee, Int. J. Hydrogen Energy, 2013, 38, 4508 CrossRef CAS.
- J.-O. Shim, D.-W. Jeong, W.-J. Jang, K.-W. Jeon, B.-H. Jeon, S. Y. Cho, H.-S. Roh, J.-G. Na, C. H. Ko, Y.-K. Oh and S. S. Han, Renewable Energy, 2014, 65, 36 CrossRef CAS.
- W.-J. Jang, D.-W. Jeong, J.-O. Shim, H.-M. Kim, W.-B. Han, J. W. Bae and H.-S. Roh, Renewable Energy, 2015, 79, 91 CrossRef CAS.
- D.-W. Jeong, H.-S. Na, J.-O. Shim, W.-J. Jang and H.-S. Roh, Catal. Sci. Technol., 2015, 5, 3706 CAS.
- D.-W. Jeong, A. Jha, W.-J. Jang, W.-B. Han and H.-S. Roh, Chem. Eng. J., 2015, 265, 100 CrossRef CAS.
- A. Jha, D.-W. Jeong, J.-O. Shim, W.-J. Jang, Y.-L. Lee, C. V. Rode and H.-S. Roh, Catal. Sci. Technol., 2015, 5, 2752 CAS.
- A. Jha, D.-W. Jeong, W.-J. Jang, Y.-L. Lee and H.-S. Roh, Int. J. Hydrogen Energy, 2015, 40, 9209 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Pore size distribution of Co3O4, isotherm plots of Ni(x%)/Co3O4 catalysts. See DOI: 10.1039/c6ra11410e |
| This journal is © The Royal Society of Chemistry 2016 |


