Adsorption and corrosion inhibition properties of N-{n-[1-R-5-(quinoxalin-6-yl)-4,5-dihydropyrazol-3-yl]phenyl}methanesulfonamides on mild steel in 1 M HCl: experimental and theoretical studies†
Lukman O. Olasunkanmiabc,
Ime B. Obotd and
Eno E. Ebenso*ab
aDepartment of Chemistry, School of Mathematical and Physical Sciences, Faculty of Agriculture, Science and Technology, North-West University (Mafikeng Campus), Private Bag X2046, Mmabatho 2735, South Africa. E-mail: Eno.Ebenso@nwu.ac.za; Fax: +27 183892052; Tel: +27 183892050/2051
bMaterial Science Innovation and Modelling (MaSIM) Research Focus Area, Faculty of Agriculture, Science and Technology, North-West University (Mafikeng Campus), Private Bag X2046, Mmabatho 2735, South Africa
cDepartment of Chemistry, Faculty of Science, Obafemi Awolowo University, Ile-Ife, 220005, Nigeria
dCentre of Research Excellence in Corrosion, Research Institute, King Fahd University of Petroleum and Minerals, Dhahran 31261, Kingdom of Saudi Arabia
First published on 22nd August 2016
Abstract
Six quinoxalinyl-dihydropyrazolyl-phenyl-methanesulfonamides were investigated for their adsorption characteristics and inhibition of mild steel corrosion in 1 M HCl medium. Tafel polarization measurements revealed that all the studied compounds are mixed-type inhibitors. Electrochemical impedance spectroscopy showed that the compounds form a pseudo-capacitive protective film on mild steel surface and protect the steel from direct acid attack. The inhibitors adsorb on mild steel in 1 M HCl via competitive physisorption and chemisorption mechanisms and their adsorption obeyed the Langmuir adsorption isotherm model. UV-vis spectra confirmed that the inhibitors interact with mild steel in solution to form Fe-inhibitor complexes. Scanning electron microscope (SEM) images also confirmed the protective efficacy of the studied compounds on mild steel in the acid. Quantum chemical calculations and quantitative structure activity relationship (QSAR) studies proposed good correlations between molecular quantum chemical descriptors and experimental inhibition efficiencies. Descriptors for protonated species correlate better than those of neutral species. Adsorption of the studied molecules was simulated on Fe(110) surface and the binding energies derived from molecular dynamics simulations corroborate experimental results. Compounds in which the sulfonamido group is attached to position 3 on the phenyl ring showed higher corrosion inhibition activities.
1 Introduction
Corrosion of metals is still considered a foremost source of economic and safety concerns to industries. This is in spite of copious research progress that has been documented in corrosion science and technology.1 Corrosion tends to occur in almost all aqueous environments,2 but the process is grievously shaped up in more aggressive media such as those encountered in oil and gas industries. Oil and gas processing and allied activities such as pipeline cleaning, pipeline/acid descaling and oil-well acidizing involve the use of mineral acids, most especially hydrochloric and sulfuric acids.3 Acid washing, matrix acidizing and fracture acidizing involve pumping of acid (usually hydrochloric acid) into the well with the aim of improving well productivity or injectivity.4,5 These unavoidable production activities unfortunately expose the metal alloys that make up the oil well and pipelines to acid corrosion.An ample number of studies in corrosion science researches focus on mild steel corrosion and prevention.6–15 The popularity of mild steel in this regards is connected with its wide usage, owing to its relatively high mechanical strength and low cost.6–10 As a type of carbon steel, mild steel also has advantages of ease of fabrication, availability and weldability over many other metal alloys.16,17 However, mild steel is highly susceptible to corrosion in common aqueous environments, especially acidic solutions.18,19 A comparative analysis of cost and efficiencies of known corrosion control and prevention methods puts the use of corrosion inhibitors at a rational edge.1 Apart from the fact that many corrosion inhibitors can be synthesized from relatively cheap materials,1 their utilization proffers a convenient approach of repressing metal corrosion.20 Inhibitors can be injected into production channels in situ without necessarily disrupting the on-going production process.20,21 Many applications involve the use of corrosion inhibitors in conjunction with other methods such as coating and cathodic protection to achieve more enhanced protection efficiency.1
Oxidizing inhibitors such as chromates and nitrates were popular in the earlier stage of corrosion inhibition studies. The major drawbacks in using these materials include the poisonous nature of chromates. In addition, the use of these compounds below some critical concentrations can favour localized corrosion and the resulting effects may be worse than no inhibitor at all.22 Organic compounds that contain π electron systems and heteroatoms such as N, O, S and P have been reported to exhibit good protection efficiencies. In a review on promising novel corrosion inhibitors, Gece23 reported the advantages of using drugs, chemical medicines or biologically active compounds as corrosion inhibitors. The central benefit of using drug-like compounds as corrosion inhibitors is their fair non-toxicity and less detrimental effects on the environment.23
Many drugs and biologically important compounds contain quinoxalines24–29 and sulfonamides29–33 as essential units or fragments. These compounds have been reported to exhibit a wide spectrum of biological activities, including antitumor, antiepileptic, antibiotic and anticancer activities.24–35 The inhibitive effects of quinoxaline9,36–46 and sulfonamide47–50 derivatives on metal corrosion have also been established. The inhibition potentials of organic compounds mainly depend on their ability to adsorb on metallic surfaces and form protective films. The modes of adsorption and corrosion inhibition potentials are influenced by many factors, which include the electronic structure, steric hindrance, π-electron network, and electron density at donor/acceptor sites of the inhibitor. For this reason, investigations of the mode of adsorption and corrosion inhibition properties of new organic compounds that are promising corrosion inhibitors appear to be a continuous and dynamic area of research.
In the present study, six quinoxalinyl-dihydropyrazolyl-phenyl-methanesulfonamides (Fig. 1) namely, N-(2-(1-propanoyl-5-(quinoxalin-6-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)methanesulfonamide (MS-2-PQPP), N-(3-(1-propanoyl-5-(quinoxalin-6-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)methanesulfonamide (MS-3-PQPP), N-(4-(1-propanoyl-5-(quinoxalin-6-yl)-4,5-dihydropyrazol-3-yl)phenyl)methanesulfonamide (MS-4-PQPP), N-(2-(1-(methanesulfonyl)-5-(quinoxalin-6-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)methanesulfonamide (MS-2-PQPMS), N-{3-[1-(methylsulfonyl)-5-(quinoxalin-6-yl)-4,5-dihydro-1H-pyrazol-3-yl]phenyl}methanesulfonamide (MS-3-PQPMS), and N-(4-(1-(methanelsulfonyl)-5-(quinoxalin-6-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)methanesulfonamide (MS-4-PQPMS) were investigated for their adsorption and corrosion inhibition properties on mild steel in 1 M HCl solution. The selection of these compounds as potential corrosion inhibitors in this study is premised on their drug-likeness, low toxicity,23 and the presence of moieties (quinoxaline and sulfonamide) with proven metal protection abilities.36–50 The quinoxaline derivatives were divided into two groups (MS-n-PQPP and MS-n-PQPMS groups (n = 2, 3, 4)) based on some levels of similarity and specific variation in their molecular structure and substituent functional groups. Experimental electrochemical studies were carried out using Tafel polarization and electrochemical impedance spectroscopy (EIS) techniques. Ultraviolet-visible (UV-vis) spectroscopic technique was used to substantiate metal–inhibitor interactions. Scanning electron microscopic images of the surfaces of mild steel specimens immersed in the (non)inhibitior-containing acid solutions were taken and reported. Theoretical quantum chemical calculations and molecular dynamic simulation studies were carried out on the studied molecules to corroborate experimental results.
It is important to mention that the compounds studied in this work are being considered as corrosion inhibitors for the first time. More so, works that employ this kind of detailed theoretical studies presented in the present work to support experimentations are scanty. To the best of our knowledge this is also the first time proton affinity (PA) of organic compounds is being proven as a substantial quantum chemical descriptor in the formulation of quantitative structure activity relationship (QSAR) empirical equations for corrosion inhibitors.
2 Experimental details
2.1 Mild steel specimens
The elemental compositions (in weight percent) of the mild steel sheets used for the corrosion tests are carbon (0.17%), manganese (0.46%), silicon (0.26%), sulfur (0.017%), phosphorus (0.019%), and balance Fe. For the purpose of electrochemical studies, mild steel sheets were cut into 1 cm × 1 cm dimension, glued to a connecting wire using aluminium foil tape, and embedded in a silico-rubber mould with the aid of epoxy resin. The fabricated mild steel utilized as the working electrode (WE) has an exposed area of 1 cm2, necessary to ensure uniform contact area for electrochemical reactions.The surface of the mild steel WE was mechanically abraded on Struers MD Piano 220 (size: 200 dia.) mounted on a Struers LaboPol-1 machine to remove traces of epoxy resin from the surface. The mild steel surface was ground with SiC paper of graded grit sizes ranging from 600 to 1200 to obtain a polished surface free of pre-experimental corrosion products. The surface was then washed with water, degreased in acetone, washed with distilled water again, before finally dried with clean white towel paper. All electrochemical experiments were carried out using a freshly surface pre-treated mild steel WE.
2.2 Aggressive electrolyte solutions
Hydrochloric acid of 32% assay was commercially obtained from Promark chemicals, South Africa, and diluted with distilled water to prepare the 1 M HCl solution (pH = 0) used for the experiments. The quinoxaline derivatives were obtained from Vitas-M Laboratory, Ltd., Apeldoorn the Netherlands; Life Chemicals Inc., Munich, Germany; Enamine Ltd., New Jersey, United States of America, and ChemBridge Corporation, San Diego, United States of America and used without further purifications. Concentrations of the inhibitor solutions used for the experiments range from 5 to 100 ppm.2.3 Electrochemical measurements
All electrochemical measurements were carried out on the Autolab PGSTAT 302N equipped with a three-electrode electrochemical cell system comprising Ag/AgCl in 3 M KCl as the reference electrode (RE), platinum rod as the counter electrode (CE), and mild steel WE. The Autolab PGSTAT 302N equipment was driven by the Nova 1.10.1.9 software. The adopted steps for the electrochemical set-up and measurements have been successfully utilized in previous work.7,17,45,51–54 A period of 30 min was allowed for the electrochemical system to reach the steady open circuit potential (OCP) or corrosion potential (Ecorr) after immersion of the WE before each electrochemical perturbation. This period was considered sufficient for OCP stabilization for an electrochemical system consisting of mild steel in acidic medium.45,52–62Tafel polarization curves were obtained by sweeping the potential up to ±100 mV63,64 with respect to the Ecorr at the scan rate of 1 mV s−1.64–67 The EIS measurements were conducted at the Ecorr by passing AC-signals through the electrochemical system and analyzing the frequency response between 100 kHz and 100 MHz at 5 mV peak-to-peak amplitude.53 All electrochemical experiments were conducted under aerated unstirred conditions at 303 K.
Corrosion inhibition efficiency (% IEP) was calculated from the Tafel polarization measurements as:45,55,59,60,68–71
 | (1) |
 | (2) |
 | (3) |
2.4 UV-vis spectroscopic studies
The UV-vis spectroscopic technique is often used in corrosion inhibition studies to inform the formation inhibitor–metal complex.54,55,73,74 In order to confirm possible formation of inhibitor–Fe complexes, UV-vis absorption spectra of 1 M HCl solutions containing 100 ppm of each quinoxaline derivative were recorded before and after 3 h of mild steel immersion.2.5 Surface morphology studies
Mild steel specimens with freshly pre-treated surface were immersed in 1 M HCl solutions without and with 100 ppm of the studied compounds. The specimens were retrieved after 3 h and the surfaces were examined using the quanta FEG 250 environmental scanning electron microscope (ESEM) equipment obtained from IMP Scientific & Precision (PTY) Ltd. Boksburg, Gauteng, South Africa.2.6 Quantum chemical calculations
Geometry optimizations and vibrational frequency calculations were carried out on the neutral and singly protonated forms of the studied quinoxaline derivatives. Geometry optimizations were performed without symmetry constraint using the density functional theory (DFT) method. Hybrid functional, B3LYP comprising the Becke’s three parameter exchange functional75 and Lee–Yang–Parr correlation functional76,77 was used in conjunction with 6-31G(d) basis set for all the calculations. The B3LYP functional has been widely used in literature in conjunction with different Pople’s basis sets to produce satisfactory geometries at relatively less computational cost.78–81 All the possible sites of protonation were considered for the singly protonated species in order to identify the most favourable site of protonation. The reason for considering the protonated species is premised on the possibility of these compounds to undergo protonation in acidic medium such that the protonated species could take part in the corrosion inhibition process. Optimized structures were confirmed to correspond to true energy minima conformers by the absence of imaginary vibrational frequencies. All the calculations were carried out in the gas phase with the aid of Gaussian 09W (revised version D.01) software.82The frontier molecular orbital (FMO) energy parameters including energy of the highest occupied molecular orbital (EHOMO) and energy of the lowest unoccupied molecular orbital (ELUMO) were obtained for both the neutral and protonated forms of the inhibitor molecules. Other relevant parameters such absolute hardness, η and absolute electronegativity, χ were calculated using the respective equations:83,84
 | (4) |
 | (5) |
The fraction of electrons transferred (ΔN) from the inhibitor (donating) molecule to the metallic (Fe) atom (acceptor) was calculated according to the equation:85
 | (6) |
2.7 Molecular dynamics (MD) simulations
Molecular dynamic (MD) simulations were carried out to model the adsorption behaviour of the studied inhibitors on iron (Fe) surface. The simulations were used to compute the binding or interaction energies of the lowest configuration interactions between the inhibitor molecules and clean iron surface. The simulations were carried out by using the forcite module in the Material Studio software 7.0 from BIOVIA-Accelrys, USA. The Fe was cleaved along (110) plane and a slab of 5 Å was employed. The Fe(110) surface was used for the simulations because it provides an adequate representation of the Fe surface with sufficient stability and moderate atom density.41,87,88 The Fe(110) plane has been successfully applied in the literature to describe the interactions between inhibitor molecules and the Fe surface.41,45,87–94 The Fe(110) plane was enlarged to a (10 × 10) super cell to provide a large surface for the interaction of the inhibitors. A vacuum slab with 30 Å thickness was built above the Fe(110) plane. The simulations were performed in NVT canonical ensemble at 298 K with a time step of 1.0 fs and a total simulation time of 500 ps using an Anderson thermostat.The condensed-phase optimized molecular potentials for atomistic simulation studies (COMPASS) force field was used to optimize the structures of all components of the system of interest. The COMPASS force field is an ab initio force field that enables accurate and simultaneous predictions of gas-phase properties (structural, conformational, vibrational, etc.) and condensed-phase (equation of state, cohesive energies, interaction energies, etc.) for a broad range of organic molecules, inorganic molecules and metals.95–99
The interaction energy (Eint) of molecules with Fe surface was obtained using the equation:
| Eint = Etotal − (Esurface + Emolecule) | (7) |
3 Results and discussion
3.1 Electrochemical studies
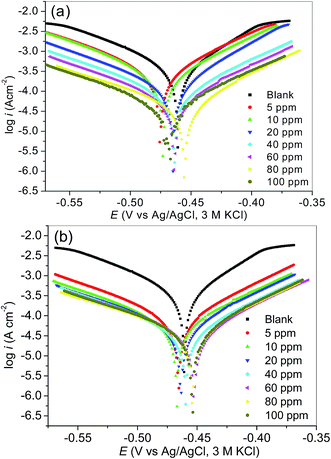 | ||
| Fig. 2 Tafel plots for mild steel in 1 M HCl without and with various concentrations of (a) MS-2-PQPP, and (b) MS-2-PQPMS, as representative Tafel plots for the studied compounds. | ||
Kinetic parameters for the corrosion reactions in the absence and presence of different concentrations of the inhibitors were obtained from the Tafel plots by extrapolating the linear Tafel regions to the corrosion potential. The results obtained via Tafel extrapolations are listed in Table 1. The observed shift in Ecorr of the inhibitor containing system relative to the blank is generally less than 85 mV for all the studied compounds. This suggests that the studied compounds might be mixed-type inhibitors.55,65,100–102 In other words, the compounds inhibit both the anodic (mild steel dissolution) reaction and the cathodic reactions. The cathodic reactions in this case may include hydrogen gas evolution (i.e. 2H+ + 2e− → H2O) and oxygen reduction (i.e. 4H+ + O2 + 4e− → 2H2O), since the experiment was conducted in aerated acidic solution. However, the oxygen reduction may not be prominent under the prevailing experimental conditions because it has been reported that the effect of dissolved oxygen on iron corrosion in acid is only more significant at pH greater than 4.103 Besides, the variation in the Ecorr seems negligible with the maximum shift of about ±17 mV. This observation suggests that the addition of the studied inhibitors does not (or only weakly) perturb the mild steel electrode surface.104 Such a small shift in the Ecorr has also been attributed to the geometric blocking of the active sites on the steel surface by the inhibitor molecules.105,106
| Compound | Conc./ppm | −Ecorr/mV | icorr/μA cm−2 | βa/mV dec−1 | βc/mV dec−1 | % IEP |
|---|---|---|---|---|---|---|
| Blank | — | 460.60 | 420.41 | 89.34 | 65.27 | — |
| MS-2-PQPP | 5 | 475.46 | 318.55 | 93.84 | 77.90 | 24.19 |
| 10 | 473.01 | 251.66 | 88.54 | 75.81 | 40.11 | |
| 20 | 464.90 | 137.89 | 96.81 | 59.76 | 67.20 | |
| 40 | 463.73 | 95.73 | 101.36 | 76.51 | 77.22 | |
| 60 | 463.72 | 70.20 | 102.01 | 76.58 | 83.29 | |
| 80 | 468.43 | 50.11 | 105.91 | 81.71 | 88.08 | |
| 100 | 455.35 | 51.34 | 116.70 | 71.85 | 87.78 | |
| MS-3-PQPP | 5 | 463.98 | 142.63 | 97.59 | 73.44 | 66.06 |
| 10 | 457.67 | 62.37 | 97.00 | 67.40 | 85.16 | |
| 20 | 461.61 | 54.87 | 91.42 | 74.03 | 86.94 | |
| 40 | 456.72 | 55.71 | 111.01 | 71.14 | 86.74 | |
| 60 | 446.76 | 40.69 | 112.77 | 70.54 | 90.32 | |
| 80 | 445.78 | 42.57 | 126.68 | 70.87 | 89.87 | |
| 100 | 440.36 | 41.35 | 137.58 | 68.03 | 90.16 | |
| MS-4-PQPP | 5 | 465.03 | 148.12 | 130.50 | 95.46 | 64.75 |
| 10 | 463.62 | 120.81 | 114.64 | 84.47 | 71.25 | |
| 20 | 462.93 | 98.12 | 121.64 | 83.28 | 76.65 | |
| 40 | 457.26 | 70.76 | 110.17 | 71.31 | 83.16 | |
| 60 | 457.30 | 50.36 | 99.82 | 67.95 | 88.02 | |
| 80 | 436.70 | 46.39 | 130.28 | 71.25 | 88.96 | |
| 100 | 446.57 | 41.50 | 122.28 | 68.82 | 90.12 | |
| MS-2-PQPMS | 5 | 465.56 | 89.66 | 94.52 | 73.95 | 78.66 |
| 10 | 467.08 | 64.91 | 96.26 | 74.48 | 84.55 | |
| 20 | 461.70 | 59.02 | 106.98 | 76.13 | 85.96 | |
| 40 | 459.43 | 42.86 | 98.11 | 75.83 | 89.80 | |
| 60 | 452.64 | 42.62 | 111.11 | 74.89 | 89.86 | |
| 80 | 453.96 | 45.89 | 120.66 | 77.68 | 89.08 | |
| 100 | 453.77 | 45.36 | 114.33 | 74.23 | 89.20 | |
| MS-3-PQPMS | 5 | 460.43 | 69.86 | 97.47 | 70.14 | 83.38 |
| 10 | 455.11 | 55.91 | 96.89 | 62.17 | 86.70 | |
| 20 | 451.55 | 62.18 | 111.60 | 74.69 | 86.23 | |
| 40 | 463.79 | 61.68 | 115.64 | 95.61 | 85.32 | |
| 60 | 443.09 | 42.83 | 115.71 | 67.38 | 89.81 | |
| 80 | 459.91 | 48.15 | 111.61 | 91.19 | 88.54 | |
| 100 | 454.09 | 41.20 | 109.76 | 84.44 | 90.20 | |
| MS-4-PQPMS | 5 | 456.99 | 131.53 | 96.36 | 71.75 | 68.70 |
| 10 | 458.81 | 70.27 | 104.07 | 67.77 | 83.28 | |
| 20 | 454.72 | 69.87 | 109.05 | 71.62 | 83.37 | |
| 40 | 455.70 | 44.17 | 104.41 | 75.84 | 89.49 | |
| 60 | 447.36 | 45.38 | 115.74 | 70.34 | 89.20 | |
| 80 | 454.99 | 45.19 | 110.94 | 73.80 | 89.25 | |
| 100 | 450.79 | 38.47 | 115.60 | 72.06 | 90.84 | |
The values of the Tafel slopes, βa and βc vary slightly with concentrations of the inhibitors, but without a simple definite pattern. However, the values of βa and βc in the presence of the inhibitors are generally higher than those of the blank. The variation in the values of the Tafel slopes with change in the concentration of the inhibitors also seems more apparent for βa than βc. These observations suggest the formation of some inhibitor complexes of Fe in the low and higher oxidation states on the mild steel electrode surface.9,107 This is suggestive of more inhibitive actions of the compounds on the anodic reaction than the cathodic one.
The corrosion current density, icorr decreases with increase in concentration of the inhibitors leading to increase in inhibition efficiency (% IEP). The % IEP of MS-2-PQPP increases from 24.19% at 5 ppm up to 88.08% at 80 ppm. Further increase in concentration does not show any significant effect on inhibition efficiency. The trend of the inhibition performance of MS-3-PQPP is such that there is an apparent increase in inhibition efficiency as the concentration of the inhibitor increases from 5 to 10 ppm. The values of the % IEP at 20 and 40 ppm are nearly the same. A maximum value of 90.32% was obtained at 60 ppm after which further increase in concentration does not seem to have noticeable effect on protection efficiency. The % IEP of MS-4-PQPP increases continuously as the concentration increases from 5 to 100 ppm. Similar trends were obtained for MS-2-PQPMS, MS-3-PQPMS and MS-4-PQPMS.
The increase in inhibition efficiency with increasing concentration may be attributed to increase in the number of adsorbed molecules of the inhibitor on the steel surface. The number of molecules that adsorb on the active sites on the steel surface increase with increasing concentration of the inhibitor, leading to an increase in surface coverage, and consequently, increase in inhibition efficiency. It is difficult to establish a simple trend for the protection efficiencies of the compounds but the overall performance based on the average values of % IEP within the concentration range considered in this work is MS-3-PQPP > MS-4-PQPP > MS-2-PQPP; and MS-3-PQPMS > MS-2-PQPMS > MS-4-PQPMS.
The Nyquist plots show single depressed semicircles corresponding to one time-constant in the Bode plots. The depression of a Nyquist semicircle is attributed to frequency dispersion effects that may result from roughness of the electrode surface and/or other forms of interfacial phenomena.109,110 The depressed one time-constant semicircular Nyquist profiles show capacitive behaviour at low frequencies, which suggests that the corrosion of mild steel in the studied media is controlled by a charge transfer process. The diameters of the Nyquist semicircles in the presence of the inhibitors are generally larger than that of the uninhibited system. This implies the mild steel/electrolyte systems exhibit higher impedance to flow of charges in the presence of the inhibitors. This is indicative of the inhibitive effect of the studied compounds.
The EIS spectra were fitted to the equivalent circuit model of the form in Fig. 3c. The values of electrochemical parameters obtained from the EIS spectra are presented in Table 2. The Rct values are higher for the inhibitor-containing systems than the blank, which implies that the resistance of the steel/electrolyte systems to the charge transfer process is enhanced by the inhibitor molecules. These observations are attributable to the formation of a protective film of the inhibitor molecules on the steel surface. The protective film of the inhibitor reduces direct contact of the steel with the aggressive acid solution. All the studied compounds show appreciable values of % IEI, which are comparable to results obtained from the Tafel polarization measurements. A maximum value of % IEI is attained at certain concentration for each compound. Similar observations were recorded from the polarization experiments (vide supra Section 3.1.1). The lower values of the constant phase element (CPE), Y0 in the presence of the inhibitors compared to the blank indicate that the inhibitor molecules adsorbed on the steel surface to form a protective layer. The values of the CPE exponent, n are close to unity, indicating the pseudo-capacitive characteristics of the electrode/electrolyte systems. The values of n are also indicative of the degree of heterogeneity of the electrode surface. The slightly lower values of n for the inhibited systems compared to the blank suggest that the steel surface is relatively more heterogeneous, which may be due to non-uniform adsorption of the inhibitor molecules on the steel surface.
| Compound | Conc./ppm | Rs/Ω cm2 | Rct/Ω cm2 | Y0/μS sn cm−2 | n | |θ|/° | |S| | % IEI |
|---|---|---|---|---|---|---|---|---|
| Blank | — | 1.01 | 28.20 | 157 | 0.893 | 60.59 | 0.64 | — |
| MS-2-PQPP | 5 | 2.65 | 42.6 | 124 | 0.886 | 54.68 | 0.66 | 34.04 |
| 10 | 2.67 | 54.6 | 124 | 0.880 | 57.12 | 0.70 | 48.53 | |
| 20 | 1.51 | 86.8 | 110.0 | 0.873 | 64.52 | 0.72 | 67.51 | |
| 40 | 3.64 | 190 | 71.9 | 0.877 | 64.45 | 0.78 | 85.21 | |
| 60 | 2.76 | 255 | 61.6 | 0.869 | 67.45 | 0.79 | 88.98 | |
| 80 | 2.89 | 337 | 67.6 | 0.848 | 67.08 | 0.79 | 91.66 | |
| 100 | 2.93 | 332 | 69.9 | 0.847 | 67.12 | 0.79 | 91.54 | |
| MS-3-PQPP | 5 | 3.04 | 125 | 78.8 | 0.878 | 63.10 | 0.76 | 77.52 |
| 10 | 2.66 | 259 | 62.1 | 0.883 | 68.90 | 0.82 | 89.15 | |
| 20 | 3.18 | 302 | 54.2 | 0.891 | 69.24 | 0.83 | 90.70 | |
| 40 | 3.48 | 304 | 58.4 | 0.868 | 67.27 | 0.80 | 90.76 | |
| 60 | 3.06 | 431 | 59.1 | 0.862 | 69.38 | 0.82 | 93.48 | |
| 80 | 2.99 | 428 | 57.1 | 0.855 | 68.86 | 0.81 | 93.44 | |
| 100 | 2.17 | 459 | 57.5 | 0.867 | 71.74 | 0.84 | 93.88 | |
| MS-4-PQPP | 5 | 19.60 | 134 | 77.7 | 0.878 | 42.60 | 0.53 | 79.03 |
| 10 | 14.60 | 161 | 70.3 | 0.887 | 49.44 | 0.61 | 82.55 | |
| 20 | 17.30 | 196 | 63.1 | 0.879 | 49.24 | 0.60 | 85.66 | |
| 40 | 7.35 | 255 | 61.8 | 0.888 | 61.75 | 0.76 | 88.98 | |
| 60 | 2.14 | 325 | 57.5 | 0.884 | 71.42 | 0.84 | 91.35 | |
| 80 | 2.96 | 431 | 68.8 | 0.863 | 69.41 | 0.82 | 93.48 | |
| 100 | 2.91 | 436 | 66.0 | 0.861 | 69.86 | 0.83 | 93.56 | |
| MS-2-PQPMS | 5 | 3.17 | 178.00 | 71.98 | 0.874 | 65.00 | 0.77 | 84.21 |
| 10 | 2.54 | 234.00 | 80.85 | 0.856 | 66.83 | 0.78 | 87.99 | |
| 20 | 3.48 | 282.00 | 66.75 | 0.854 | 65.80 | 0.78 | 90.04 | |
| 40 | 2.28 | 365.00 | 61.56 | 0.874 | 70.86 | 0.83 | 92.30 | |
| 60 | 2.43 | 378.00 | 57.82 | 0.868 | 61.08 | 0.76 | 92.57 | |
| 80 | 2.33 | 420.00 | 60.00 | 0.854 | 69.91 | 0.81 | 93.30 | |
| 100 | 2.80 | 384.00 | 64.90 | 0.861 | 69.37 | 0.82 | 92.68 | |
| MS-3-PQPMS | 5 | 2.26 | 229.00 | 63.24 | 0.876 | 66.96 | 0.79 | 87.73 |
| 10 | 3.07 | 247.00 | 71.63 | 0.864 | 52.03 | 0.64 | 88.62 | |
| 20 | 2.18 | 293.00 | 61.80 | 0.869 | 57.24 | 0.72 | 90.41 | |
| 40 | 3.20 | 401.00 | 54.66 | 0.875 | 54.42 | 0.67 | 92.99 | |
| 60 | 2.85 | 395.00 | 55.67 | 0.873 | 70.17 | 0.83 | 92.89 | |
| 80 | 2.51 | 409.00 | 67.47 | 0.855 | 69.67 | 0.82 | 93.13 | |
| 100 | 2.52 | 425.00 | 64.50 | 0.857 | 69.91 | 0.82 | 93.39 | |
| MS-4-PQPMS | 5 | 2.96 | 91.39 | 92.84 | 0.895 | 61.79 | 0.75 | 69.25 |
| 10 | 1.98 | 214.00 | 67.38 | 0.869 | 68.82 | 0.79 | 86.87 | |
| 20 | 2.74 | 256.00 | 68.93 | 0.872 | 67.70 | 0.80 | 89.02 | |
| 40 | 2.38 | 387.00 | 50.28 | 0.885 | 71.81 | 0.84 | 92.74 | |
| 60 | 2.59 | 393.00 | 56.88 | 0.872 | 70.41 | 0.82 | 92.85 | |
| 80 | 2.68 | 398.00 | 61.72 | 0.876 | 70.81 | 0.83 | 92.94 | |
| 100 | 2.41 | 468.00 | 59.06 | 0.869 | 71.39 | 0.84 | 94.00 | |
The magnitudes of the maximum phase angle, |θ| and slope of the linear portion of the Bode impedance modulus (at intermediate frequencies), |S| are also listed in Table 2. The results show that |θ| is closer to 90°, while |S| is closer to 1 at higher concentrations of the inhibitors compared to the blank system. This suggests that the electrode/electrolyte interface behaves closer to an ideal capacitor at higher concentrations of the inhibitors. This also implies that the inhibitor molecules adsorbed on the steel surface to form a pseudo-capacitive protective film.
3.2 Adsorption isotherms
Corrosion inhibition by organic molecules usually results from the adsorption of the inhibitor molecules on the metallic surface. Molecules of inhibitor tend to displace water molecules from the surface of the metal in a reversible process of the form:45| Inh(sol) + xH2O(ads) ⇌ Inh(ads) + xH2O(sol) | (8) |
 | (9) |
Representative isotherm plots are shown in Fig. 4 for MS-2-PQPP and MS-2-PQPMS. The isotherm plots for other compounds (Fig. S4†) are also similar. The correlation coefficient (R2) values of the plots are unity (1.000), while the slopes of the plots are near unity, i.e. 0.98 (MS-2-PQPP), 1.06 (MS-3-PQPP), 1.05 (MS-4-PQPP), 1.07 (MS-2-PQPMS), 1.07 (MS-3-PQPMS) and 1.05 (MS-4-PQPMS). These observations infer the conformity of the data to the linearized Langmuir adsorption isotherm. The values of Kads and ΔGads are listed in Table 3. The high values of Kads obtained for the studied compounds imply that the displacement of water molecules from the steel surface by the inhibitor molecules, and consequently the adsorption of the inhibitor molecules on the steel surface is a favourable process, and the compounds adsorb strongly on the steel surface. The negative values of ΔGads also suggest spontaneous adsorption of the inhibitor molecules on the steel surface. It can be inferred from the range of values of ΔGads in Table 3 that the mode of adsorption of MS-2-PQPP involves competitive physisorption and chemisorption, while the remainder of the compounds adsorb mainly via charge sharing (chemisorption). The trend of the values of Kads and ΔGads is such that MS-3-PQPP > MS-4-PQPP > MS-2-PQPP, and MS-3-PQPMS > MS-2-PQPMS > MS-4-PQPMS, which is the same as the relative order of the % IE.
 | ||
| Fig. 4 Langmuir adsorption isotherms for MS-2-PQPP on mild steel in 1 M HCl (surface coverage data were taken from the EIS measurements). | ||
| Inhibitor | Kads (×103) | −ΔGads (kJ mol−1) |
|---|---|---|
| MS-2-PQPP | 44.15 | 37.08 |
| MS-3-PQPP | 397.80 | 42.62 |
| MS-4-PQPP | 226.20 | 41.20 |
| MS-2-PQPMS | 692.90 | 44.02 |
| MS-3-PQPMS | 791.10 | 44.35 |
| MS-4-PQPMS | 311.69 | 42.01 |
3.3 UV-vis spectroscopic analyses
UV-vis spectroscopic analyses were carried out on the solutions of the studied inhibitor molecules and the resulting solutions after mild steel immersion for 3 h and the spectra are shown in Fig. 5. The absorption bands at 200–201 nm in the UV-vis spectra of MS-2-PQPP, MS-3-PQPP and MS-4-PQPP are due to n → π* transition. MS-2-PQPP also shows a shoulder-like band of lower absorbance at 219 nm, while MS-3-PQPP and MS-4-PQPP both show absorption bands at 239 and 300–301 nm due to π → π* transitions and intra-molecular charge transfers (ICT) respectively. MS-2-PQPMS and MS-3-PQPMS show multiple absorption bands at 208–209, 240, 273 and 327–329 nm, which are due to n → π*, π → π* transitions, and intramolecular charge transfers (ICT). MS-4-PQPMS shows bands at 206, 242 and 285 nm, which can also be attributed to n → π*, π → π* transitions, and intramolecular charge transfers (ICT) respectively.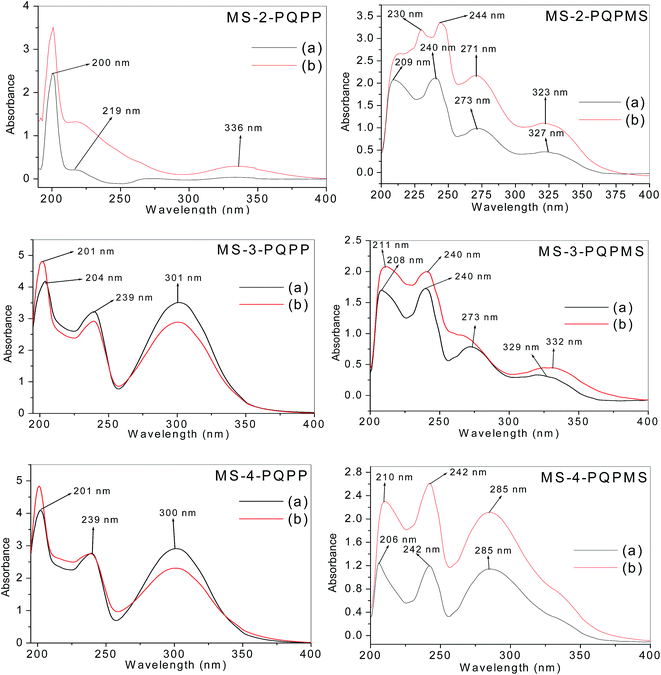 | ||
| Fig. 5 UV-vis spectra of acidic solutions of studied inhibitors (a) before, and (b) after mild steel immersion. | ||
After mild steel immersion, MS-2-PQPP shows a new band at 336 nm and the absorbances are generally higher compared to prior mild steel immersion. The major difference in the UV-vis spectra of the solutions of MS-3-PQPP and MS-4-PQPP after mild steel immersion is the increase in absorbance of the bands at 200–201 nm. MS-2-PQPMS shows an additional band at 230 nm, while the band at 240 nm is red-shifted to 244 nm, and those at 273 and 327 nm are blue-shifted to 271 and 323 nm, respectively. For MS-3-PQPMS, the bands at 208 and 329 nm shifted to longer wavelengths, appearing at 211 and 332 nm, respectively, while that at 273 nm broadens to a shoulder-like band. The band at 206 nm in the UV-vis spectrum of MS-4-PQPMS red-shifted to 210 nm after steel immersion. The changes in the spectra features after mild steel immersion can be attributed to interactions between Fe and the inhibitor molecules and possible formation of Fe–inhibitor complexes.
3.4 Surface morphology
Scanning electron microscopy (SEM) images of mild steel surfaces immersed in 1 M HCl without and with 100 ppm of the studied inhibitors are shown in Fig. 6. The surface of mild steel immersed in 1 M HCl without the inhibitors exhibit highly eroded and damaged features due to unprotected exposure to corrosive ions in the aggressive medium. Mild steel specimens retrieved from the inhibitor-containing solutions show relatively smooth surface. The noticeable roughness on these specimens is mainly due to the polishing scratches introduced to the surface during mechanical abrasion. This again is an indication that the studied compounds adsorb as protective film on mild steel surface and prevent it from direct attack by aggressive ions in the corrosive environment.3.5 Quantum chemical studies
 | ||
| Fig. 7 Optimized structures of the studied compounds (selected atomic labels were referred in the discussion of results). | ||
The HOMOs of all the molecules comprise both π- and σ-type orbitals, but are essentially dominated by π-type molecular orbitals. The HOMO electron density is distributed over the entire pyrazole and phenyl rings. In the MS-n-PQPP series, the N-atom of the methanesulfonamido group (attached to the phenyl ring) and the O-atom of the carbonyl functional group in each case are also involved in the HOMO electron density distributions. In the case of MS-n-PQPMS compounds, the HOMO is extended to the N-atom of the methanesulfonamido group (attached to the phenyl ring) and O-atoms of the methanesulfonamido group (attached to the pyrazole ring). This suggests that the interactions of the studied molecules with mild steel via π-electron donation to the vacant d-orbitals of Fe can occur predominantly around the pyrazole and phenyl rings. The HOMO orbitals around the N-atom of the methanesulfonamido group and the carbonyl O-atom are σ-type, indicating that these groups can interact with vacant p-orbitals of Fe. The S- and O-atoms of the sulfonamido group are not involved in the HOMO electron density distributions.
The LUMO electron density distributions on the other hand are mainly found on the quinoxaline moieties of the molecules. Apart from the atoms in the quinoxaline ring, the sp2 N-atom of the pyrazole ring and the C-atoms adjoining the pyrazole and phenyl rings in MS-3-PQPP are also involved in the LUMO electron density surface of the molecule. Some of the C-atoms in the phenyl ring especially those around the sulfonamido groups also contribute to the LUMO electron density distributions in MS-3-PQPP. The LUMO of MS-2-PQPMS is slightly extended to the sp2 N-atom of the pyrazole ring. The LUMO of MS-3-PQPMS involves the π-electron centre of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group adjoining the pyrazole to the phenyl ring, the sp2 N-atom of the pyrazole ring, and the C-atom of the phenyl ring adjacent to the methanesulfonamido group.
C group adjoining the pyrazole to the phenyl ring, the sp2 N-atom of the pyrazole ring, and the C-atom of the phenyl ring adjacent to the methanesulfonamido group.
Fukui indices have been widely used to identify the prospective atomic sites with which an inhibitor molecule interacts with metallic atom.38,111–114 The atomic sites with highest susceptibility to nucleophilic and electrophilic attacks were predicted by calculating f+ (for nucleophilic) and f− (for electrophilic) attacks as:45
| fk+ = ρk(N+1)(r) − ρk(N)(r) | (10) |
| fk− = ρk(N)(r) − ρk(N−1)(r) | (11) |
It is apparent from the f+ electron density surfaces that for all the inhibitor molecules, the prospective interactions of a negatively charged metallic surface with the inhibitor molecules will occur mainly through the N-atoms and the aromatic centre (C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3) in the quinoxaline ring, the sp2 N-atom (N3) in the pyrazole ring, and the π-electron centre between the C-atoms adjoining the pyrazole and phenyl rings. Some of the C-atoms (in the phenyl ring) especially those in the neighbourhood of the methanesulfonamido group also appear to be prospective sites for nucleophilic attack. Methanesulfonamido groups are not involved in the f+ electron density distributions. The most probable sites for electrophilic attacks are depicted with the f− graphical isosurfaces. In all the inhibitor molecules, the two N-atoms in the pyrazole ring, the carbonyl oxygen, the π-electron centres on the phenyl ring (around the point of attachment of the methanesulfonamido group) are prospective sites of electrophilic attack. These observations suggest that in acidic medium where a positively charged metallic surface overlaid with negatively charged chloride ions (from HCl solution) is present, the studied inhibitors will interact essentially through the quinoxaline ring. On the other hand, positively charged metallic surface or ions can bind to the inhibitor molecules via the pyrazole ring.
C3) in the quinoxaline ring, the sp2 N-atom (N3) in the pyrazole ring, and the π-electron centre between the C-atoms adjoining the pyrazole and phenyl rings. Some of the C-atoms (in the phenyl ring) especially those in the neighbourhood of the methanesulfonamido group also appear to be prospective sites for nucleophilic attack. Methanesulfonamido groups are not involved in the f+ electron density distributions. The most probable sites for electrophilic attacks are depicted with the f− graphical isosurfaces. In all the inhibitor molecules, the two N-atoms in the pyrazole ring, the carbonyl oxygen, the π-electron centres on the phenyl ring (around the point of attachment of the methanesulfonamido group) are prospective sites of electrophilic attack. These observations suggest that in acidic medium where a positively charged metallic surface overlaid with negatively charged chloride ions (from HCl solution) is present, the studied inhibitors will interact essentially through the quinoxaline ring. On the other hand, positively charged metallic surface or ions can bind to the inhibitor molecules via the pyrazole ring.
| Neutral species | ||||||
|---|---|---|---|---|---|---|
| Parameter | MS-2-PQPP | MS-3-PQPP | MS-4-PQPP | MS-2-PQPMS | MS-3-PQPMS | MS-4-PQPMS |
| a Data are reported for the most stable protonated species; respective sites of protonation are indicated as subscripts e.g. (N1), (N2) etc. based on atom labeling in Fig. 6, where O1 is the carbonyl oxygen.b PA was calculated as previously reported.45 | ||||||
| ELUMO/eV | −2.243 | −1.942 | −1.946 | −1.974 | −2.051 | −1.981 |
| EHOMO/eV | −6.124 | −5.983 | −5.734 | −6.329 | −6.444 | −6.004 |
| ΔEL–H/eV | 3.881 | 4.041 | 3.789 | 4.355 | 4.393 | 4.023 |
| χ/eV | 4.183 | 3.963 | 3.840 | 4.152 | 4.248 | 3.993 |
| η/eV | 1.941 | 2.020 | 1.894 | 2.178 | 2.196 | 2.012 |
| ΔN | 0.726 | 0.752 | 0.834 | 0.654 | 0.626 | 0.748 |
| μ/Debye | 3.816 | 5.493 | 4.505 | 5.930 | 3.641 | 1.049 |
| Protonated speciesa | ||||||
|---|---|---|---|---|---|---|
| Parameters | MS-2-PQPP-H+(N3) | MS-3-PQPP-H+(O1) | MS-4-PQPP-H+(N2) | MS-2-PQPMS-H+(N3) | MS-3-PQPMS-H+(N1) | MS-4-PQPMS-H+(N1) |
| ELUMO/eV | −6.085 | −5.323 | −6.962 | −6.123 | −6.971 | −6.971 |
| EHOMO/eV | −9.246 | −8.948 | −7.947 | −9.216 | −8.280 | −8.041 |
| ΔEL–H/eV | 3.160 | 3.626 | 0.985 | 3.093 | 1.309 | 1.070 |
| χ/eV | 7.665 | 7.136 | 7.455 | 7.670 | 7.625 | 7.506 |
| η/eV | 1.580 | 1.813 | 0.493 | 1.547 | 0.654 | 0.535 |
| ΔN | −0.210 | −0.037 | −0.462 | −0.217 | −0.478 | −0.473 |
| μ/Debye | 4.011 | 7.772 | 21.714 | 6.501 | 15.592 | 19.579 |
| PAb/kJ mol−1 | 966.68 | 938.85 | 949.61 | 948.28 | 945.57 | 947.57 |
In the present study, however, an attempt to correlate individual quantum chemical parameters (Table 4) with average corrosion inhibition efficiencies of the studied molecules could not yield a direct trend. In a case like this, relationships between inhibition efficiencies and quantum chemical parameters are often described by using composite theoretical data. Linear and/or non-linear equations that relate average corrosion inhibition efficiencies with multiple quantum chemical descriptors are obtainable with the aid of quantitative structure activity relationship (QSAR) analysis. QSAR has been successfully used in literature to examine the dependence of average corrosion inhibition strengths of molecules on a composite index of more than one quantum chemical descriptor.47,119,120 Several attempts were made to correlate different linear combinations of relevant parameters with average experimental inhibition efficiencies of the molecules. QSAR analyses were performed with the aid of the XLSTAT (Microsoft Excel add-in) software121 and the results are listed in Table 5.
| Parameters | QSAR equations | R2 | MSE | RMSE |
|---|---|---|---|---|
a  ; IEpred = predicted inhibition efficiency; IEexp = experimental efficiency, n is the number of observations (n = 6). ; IEpred = predicted inhibition efficiency; IEexp = experimental efficiency, n is the number of observations (n = 6). |
||||
| Neutral species | ||||
| EHOMO, ELUMO, ΔN, μ | IE = −553.872 − 97.162EHOMO + 76.955ELUMO + 283.672ΔN − 0.476μ | 0.981 | 4.705 | 2.169 |
| ΔEL–H, χ, ΔN, μ | IE = −538.733 + 85.942ΔEL–H + 18.747χ + 277.212ΔN − 0.476μ | 0.981 | 4.719 | 2.172 |
| ΔEL–H, ΔN, μ, PA | IE = −98.640 + 58.233ΔEL–H + 158.817ΔN − 0.415μ − 0.174PA | 0.990 | 2.492 | 1.579 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Protonated species | ||||
| ΔEL–H, χ, μ, PA | IE = 708.421 + 5.056ΔEL–H + 23.748χ + 0.910μ − 0.866PA | 0.997 | 0.647 | 0.805 |
| ΔEL–H, χ, ΔN, PA | IE = 764.510 − 16.045ΔEL–H + 39.360χ + 110.713ΔN − 0.951PA | 0.998 | 0.546 | 0.739 |
| EHOMO, ELUMO, μ, PA | IE = 707.979 − 6.798ELUMO − 17.033EHOMO + 0.920μ − 0.867PA | 0.997 | 0.649 | 0.806 |
The results in Table 5 show that composite indices of quantum chemical descriptors including EHOMO, ELUMO, χ, ΔN, μ and PA correlate well with average inhibition efficiencies of the molecules. The derived QSAR equations were ranked based on nearness of correlation coefficient (R2) values to unity and small values of the mean square error (MSE) and root-mean-square error (RMSE). When the quantum chemical descriptors for the neutral molecules were used, a linear combination of ΔEL–H, ΔN, μ and PA gave the best correlation, suggesting that PA plays a role in corrosion inhibition efficiency of the molecules. The best correlation was obtained from a linear combination quantum chemical parameters of the protonated species, that is, ΔEL–H, χ, ΔN and PA giving an R2 value of 0.998, MSE and RMSE values of 0.546 and 0.739, respectively. The plot of experimental inhibition efficiencies (IEexp) versus predicted inhibition efficiencies (IEpred) (based on the best QSAR equation, IE = 764.510 − 16.045ΔEL–H + 39.360χ + 110.713ΔN − 0.951PA) is shown in Fig. 10. The results suggest that the corrosion inhibition efficiency of the studied compounds depend on the HOMO–LUMO energy gap, electronegativity, fraction of electron transferred to metal, and proton affinity, such that lower ΔEL–H and PA, as well as higher χ and ΔN will favour higher protection performance.
3.6 Molecular dynamic (MD) simulations
The lowest energy configurations for the adsorption of the studied inhibitor molecules on the Fe(110) surface obtained from MD simulations are shown in Fig. 11. All the inhibitor molecules adopt near-flat orientation on the Fe surface, which supports optimum interactions with the metallic substrate. The methyl group of the methanesulfonamido substituent in MS-2-PQPP, MS-2-PQPMS and MS-4-PQPMS does not adopt a parallel inclination with Fe(110) surface. This might reduce the degree of interactions with the metallic surface. This does not happen in the case of MS-3-PQPP and MS-3-PQPMS, which suggests a higher degree of interactions between these molecules and Fe(110) substrate.The interaction and binding energies obtained from the simulations are listed in Table 6. The results show that the order of binding energies of the inhibitor/Fe(110) systems is MS-3-PQPP/Fe(110) > MS-4-PQPP/Fe(110) > MS-2-PQPP/Fe(110), and MS-3-PQPMS/Fe(110) > MS-2-PQPMS/Fe(110) > MS-4-PQPMS/Fe(110) which is in agreement with the trends of observed inhibition strengths of the compounds.
| System | Interaction energy | Binding energy |
|---|---|---|
| Fe(110) + MS-2-PQPP | −976.908 | 976.908 |
| Fe(110) + MS-3-PQPP | −1113.337 | 1113.337 |
| Fe(110) + MS-4-PQPP | −1095.693 | 1095.693 |
| Fe(110) + MS-2-PQPMS | −868.230 | 868.230 |
| Fe(110) + MS-3-PQPMS | −877.046 | 877.046 |
| Fe(110) + MS-4-PQPMS | −846.833 | 846.833 |
4 Conclusions
Corrosion inhibition properties of six quinoxalinyl-dihydropyrazolyl-phenyl-methanesulfonamide based compounds were investigated using electrochemical, UV-vis spectroscopy, scanning electron microscopy, quantum chemical calculations, quantitative structure activity relationship, and molecular dynamic simulation studies. The following conclusions can be drawn from the studies:(i) All the studied compounds showed substantial protection performances for mild steel in hydrochloric acid medium. Their inhibition efficiencies increased with increasing concentration up to some limiting values.
(ii) All the studied compounds are mixed-type inhibitors; they inhibit both anodic mild steel dissolution and cathodic hydrogen gas evolution and/or oxygen reduction reactions.
(iii) The compounds form pseudo-capacitive protective films on mild steel surface and protect the steel from direct acid attack.
(iv) The compounds adsorbed on mild steel surface via competitive physisorption and chemisorption mechanisms and their adsorption obeyed the Langmuir adsorption isotherm model.
(v) UV-vis spectra suggested inhibitor–Fe interactions, while SEM images confirmed that the compounds protect mild steel surface from direct exposure to corrosive ions.
(vi) Quantum chemical descriptors exhibit excellent correlations with inhibition performances and QSAR equations with high correlation coefficients and minimum statistical errors were derived. QSAR analyses suggested the participation of protonated species in the corrosion inhibition process.
(vii) Binding energies derived from molecular dynamics simulations are in agreement with the trend of experimental inhibition efficiencies.
(viii) Inhibitive strengths were affected by the position of the sulfonamido group attached the phenyl ring, such that substitution at position 3 favours higher inhibition potential.
Acknowledgements
L. O. O acknowledges the NRF/Sasol Inzalo foundation for support towards his PhD program. E. E. E acknowledges financial support from NRF (South Africa) for incentive funding for rated researchers. I. B. O is grateful to the Centre of Research Excellence in Corrosion, King Fahd University of Petroleum and Minerals (KFUPM) for support.References
- V. S. Saji, Recent Pat. Corros. Sci., 2010, 2, 6–12 CrossRef CAS.
- M. G. Fontana, Corrosion engineering, Tata McGraw-Hill Education, 2005 Search PubMed.
- X. Zhang, Y. Zheng, X. Wang, Y. Yan and W. Wu, Ind. Eng. Chem. Res., 2014, 53, 14199–14207 CrossRef CAS.
- Acidizing-Treatment in Oil and Gas Operators, American Petroleum Institute, DM2014-113, 2014.
- C. Crowe, J. Masmonteil and R. Thomas, Oilfield Rev., 1992, 4, 22–40 Search PubMed.
- P. Singh, E. E. Ebenso, L. O. Olasunkanmi, I. B. Obot and M. A. Quraishi, J. Phys. Chem. C, 2016, 120, 3408–3419 CAS.
- M. E. Mashuga, L. O. Olasunkanmi, A. S. Adekunle, S. Yesudass, M. M. Kabanda and E. E. Ebenso, Materials, 2015, 8, 3607–3632 CrossRef.
- T. Peme, L. O. Olasunkanmi, I. Bahadur, A. S. Adekunle, M. M. Kabanda and E. E. Ebenso, Molecules, 2015, 20, 16004–16029 CrossRef CAS PubMed.
- L. O. Olasunkanmi, M. M. Kabanda and E. E. Ebenso, Phys. E., 2016, 76, 109–126 CrossRef CAS.
- D. De la Fuente, I. Díaz, J. Simancas, B. Chico and M. Morcillo, Corros. Sci., 2011, 53, 604–617 CrossRef CAS.
- A. Popova, E. Sokolova, S. Raicheva and M. Christov, Corros. Sci., 2003, 45, 33–58 CrossRef.
- S. Nesic, J. Postlethwaite and S. Olsen, CORROSION, 1996, 52, 280–294 CrossRef CAS.
- M. Lagrenee, B. Mernari, M. Bouanis, M. Traisnel and F. Bentiss, Corros. Sci., 2002, 44, 573–588 CrossRef CAS.
- M. Nordsveen, S. Nešic, R. Nyborg and A. Stangeland, CORROSION, 2003, 59, 443–456 CrossRef CAS.
- W.-H. Li, Q. He, S.-T. Zhang, C.-L. Pei and B.-R. Hou, J. Appl. Electrochem., 2008, 38, 289–295 CrossRef CAS.
- D. Gandy, Carbon Steel Handbook, Electric Power Research Institute Inc, California, USA, 2007 Search PubMed.
- L. C. Murulana, A. K. Singh, S. K. Shukla, M. M. Kabanda and E. E. Ebenso, Ind. Eng. Chem. Res., 2012, 51, 13282–13299 CrossRef CAS.
- D. K. Yadav, D. Chauhan, I. Ahamad and M. Quraishi, RSC Adv., 2013, 3, 632–646 RSC.
- A. A. Al-Amiery, A. A. H. Kadhum, A. B. Mohamad and S. Junaedi, Materials, 2013, 6, 1420–1431 CrossRef CAS.
- P. B. Raja and M. G. Sethuraman, Mater. Lett., 2008, 62, 113–116 CrossRef CAS.
- M. A. Migahed and I. F. Nassar, Electrochim. Acta, 2008, 53, 2877–2882 CrossRef CAS.
- E. Bardal, Corrosion and protection, Springer Science & Business Media, 2007 Search PubMed.
- G. Gece, Corros. Sci., 2011, 53, 3873–3898 CrossRef CAS.
- M. Waring, L. Wakelin and J. Lee, Biochim. Biophys. Acta, Nucleic Acids Protein Synth., 1975, 407, 200–212 CrossRef CAS.
- T. Zarnowski, Z. Kleinrok, W. Turski and S. Czuczwar, Neuropharmacology, 1993, 32, 895–900 CrossRef CAS PubMed.
- K. I. Priyadarsini, M. F. Dennis, M. A. Naylor, M. R. Stratford and P. Wardman, J. Am. Chem. Soc., 1996, 118, 5648–5654 CrossRef CAS.
- H. U. Gali-Muhtasib, M. J. Haddadin, D. N. Rahhal and I. H. Younes, Oncol. Rep., 2001, 8, 679–684 CAS.
- H. Gao, E. F. Yamasaki, K. K. Chan, L. L. Shen and R. M. Snapka, Mol. Pharmacol., 2003, 63, 1382–1388 CrossRef CAS PubMed.
- I. Awad, J. Chem. Technol. Biotechnol., 2007, 53, 227–236 CrossRef.
- D. Vullo, A. Innocenti, I. Nishimori, J. R. Pastorek, A. Scozzafava, S. Pastoreková and C. T. Supuran, Bioorg. Med. Chem. Lett., 2005, 15, 963–969 CrossRef CAS PubMed.
- T. J. Welch, W. F. Fricke, P. F. McDermott, D. G. White, M.-L. Rosso, D. A. Rasko, M. K. Mammel, M. Eppinger, M. Rosovitz and D. Wagner, PLoS One, 2007, 2, e309 Search PubMed.
- H. Yoshino, N. Ueda, J. Niijima, H. Sugumi, Y. Kotake, N. Koyanagi, K. Yoshimatsu, M. Asada and T. Watanabe, J. Med. Chem., 1992, 35, 2496–2497 CrossRef CAS PubMed.
- T. A. Msagati and M. M. Nindi, Talanta, 2004, 64, 87–100 CrossRef CAS PubMed.
- C. Bailly and J. M. Waring, Biochem. J., 1998, 330, 81–87 CrossRef CAS PubMed.
- C. Carisson, M. Johnson and B. Åkerman, Nucleic Acids Res., 1995, 23, 2413–2420 CrossRef.
- I. Obot and N. Obi-Egbedi, Mater. Chem. Phys., 2010, 122, 325–328 CrossRef CAS.
- I. Obot, N. Obi-Egbedi and N. Odozi, Corros. Sci., 2010, 52, 923–926 CrossRef CAS.
- Z. El Adnani, M. Mcharfi, M. Sfaira, M. Benzakour, A. Benjelloun and M. E. Touhami, Corros. Sci., 2013, 68, 223–230 CrossRef CAS.
- A. Zarrouk, A. Dafali, B. Hammouti, H. Zarrok, S. Boukhris and M. Zertoubi, Int. J. Electrochem. Sci., 2010, 5, 46–55 CAS.
- I. El Ouali, B. Hammouti, A. Aouniti, Y. Ramli, M. Azougagh, E. Essassi and M. Bouachrine, J. Mater. Environ. Sci., 2010, 1(N°1), 1–8 CAS.
- J. Fu, H. Zang, Y. Wang, S. Li, T. Chen and X. Liu, Ind. Eng. Chem. Res., 2012, 51, 6377–6386 CrossRef CAS.
- I. Obot and N. Obi-Egbedi, Corros. Sci., 2010, 52, 282–285 CrossRef CAS.
- M. Elayyachy, B. Hammouti, A. El Idrissi and A. Aouniti, Port. Electrochim. Acta, 2011, 29, 57–68 CrossRef CAS.
- J. Saranya, P. Sounthari, K. Parameswari and S. Chitra, Measurement, 2016, 77, 175–186 CrossRef.
- L. O. Olasunkanmi, I. B. Obot, M. M. Kabanda and E. E. Ebenso, J. Phys. Chem. C, 2015, 119, 16004–16019 CAS.
- J. Saranya, P. Sounthari, A. Kiruthuka, K. Parameswari and S. Chitra, J. Mater. Environ. Sci., 2015, 6, 425–444 Search PubMed.
- E. E. Ebenso, T. Arslan, F. Kandem
![[i with combining dot above]](https://www.rsc.org/images/entities/char_0069_0307.gif) rlı, I. Love, C. L. Öğretır, M. Saracoğlu and S. A. Umoren, Int. J. Quantum Chem., 2010, 110, 2614–2636 CrossRef CAS.
rlı, I. Love, C. L. Öğretır, M. Saracoğlu and S. A. Umoren, Int. J. Quantum Chem., 2010, 110, 2614–2636 CrossRef CAS. - T. Arslan, F. Kandemirli, E. E. Ebenso, I. Love and H. Alemu, Corros. Sci., 2009, 51, 35–47 CrossRef CAS.
- A. Samide, B. Tutunaru, C. Negrila and I. Prunaru, Spectrosc. Lett., 2012, 45, 55–64 CrossRef CAS.
- L. C. Murulana, M. M. Kabanda and E. E. Ebenso, RSC Adv., 2015, 5, 28743–28761 RSC.
- S. K. Saha, A. Dutta, P. Ghosh, D. Sukul and P. Banerjee, Phys. Chem. Chem. Phys., 2015, 17, 5679–5690 RSC.
- N. Soltani, M. Behpour, E. Oguzie, M. Mahluji and M. Ghasemzadeh, RSC Adv., 2015, 5, 11145–11162 RSC.
- S. A. Umoren, I. B. Obot and Z. M. Gasem, Ionics, 2015, 21, 1171–1186 CrossRef CAS.
- J. N. Asegbeloyin, P. M. Ejikeme, L. O. Olasunkanmi, A. S. Adekunle and E. E. Ebenso, Materials, 2015, 8, 2918–2934 CrossRef.
- Y. Sasikumar, A. Adekunle, L. Olasunkanmi, I. Bahadur, R. Baskar, M. Kabanda, I. Obot and E. Ebenso, J. Mol. Liq., 2015, 211, 105–118 CrossRef CAS.
- I. Ahamad, R. Prasad and M. Quraishi, Corros. Sci., 2010, 52, 933–942 CrossRef CAS.
- K. Ansari, M. Quraishi and A. Singh, Corros. Sci., 2014, 79, 5–15 CrossRef CAS.
- H. Elmsellem, T. Harit, A. Aouniti, F. Malek, A. Riahi, A. Chetouani and B. Hammouti, Prot. Met. Phys. Chem. Surf., 2015, 51, 873–884 CrossRef CAS.
- A. Ousslim, K. Bekkouch, A. Chetouani, E. Abbaoui, B. Hammouti, A. Aouniti, A. Elidrissi and F. Bentiss, Res. Chem. Intermed., 2014, 40, 1201–1221 CrossRef CAS.
- A. Singh, I. Ahamad, V. Singh and M. A. Quraishi, J. Solid State Electrochem., 2011, 15, 1087–1097 CrossRef CAS.
- A. K. Singh and M. Quraishi, Corros. Sci., 2011, 53, 1288–1297 CrossRef CAS.
- D. K. Yadav, M. Quraishi and B. Maiti, Corros. Sci., 2012, 55, 254–266 CrossRef CAS.
- S. A. Ali, M. T. Saeed and S. U. Rahman, Corros. Sci., 2003, 45, 253–266 CrossRef.
- H. Simillion, MSc. Thesis, Vrije Universiteit Brussel, 2012 Search PubMed.
- A. Satapathy, G. Gunasekaran, S. Sahoo, K. Amit and P. Rodrigues, Corros. Sci., 2009, 51, 2848–2856 CrossRef CAS.
- R. Patil and S. Radhakrishnan, Prog. Org. Coat., 2006, 57, 332–336 CrossRef CAS.
- R. T. Loto, C. A. Loto, A. P. I. Popoola and T. Fedotova, Arabian J. Chem., 2015 DOI:10.1016/j.arabjc.2014.12.024.
- S. Kharchouf, L. Majidi, M. Bouklah, B. Hammouti, A. Bouyanzer and A. Aouniti, Arabian J. Chem., 2014, 7, 680–686 CrossRef CAS.
- S. Umoren and I. Obot, J. Adhes. Sci. Technol., 2014, 28, 2054–2068 CrossRef CAS.
- S. Umoren, I. Obot, A. Madhankumar and Z. Gasem, J. Adhes. Sci. Technol., 2015, 29, 271–295 CrossRef CAS.
- S. A. Umoren, Z. M. Gasem and I. B. Obot, Ind. Eng. Chem. Res., 2013, 52, 14855–14865 CrossRef CAS.
- Z. Tao, S. Zhang, W. Li and B. Hou, Corros. Sci., 2009, 51, 2588–2595 CrossRef CAS.
- Y. Abboud, A. Abourriche, T. Saffaj, M. Berrada, M. Charrouf, A. Bennamara, N. Al Himidi and H. Hannache, Mater. Chem. Phys., 2007, 105, 1–5 CrossRef CAS.
- X. Li, S. Deng, H. Fu, G. Mu and N. Zhao, Appl. Surf. Sci., 2008, 254, 5574–5586 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- R. G. Parr and W. Yang, J. Am. Chem. Soc., 1984, 106, 4049–4050 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B, 1988, 37, 785 CrossRef CAS.
- O. H. O. Brovarets, R. O. Zhurakivsky and D. M. Hovorun, Phys. Chem. Chem. Phys., 2014, 16, 3715–3725 RSC.
- M. Lozynski, D. Rusinska-Roszak and H.-G. Mack, J. Phys. Chem. A, 1998, 102, 2899–2903 CrossRef CAS.
- S. P. Samijlenko, Y. P. Yurenko, A. V. Stepanyugin and D. M. Hovorun, J. Phys. Chem. B, 2010, 114, 1454–1461 CrossRef CAS PubMed.
- K. B. Wiberg, J. Comput. Chem., 2004, 25, 1342–1346 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, GAUSSIAN 09 (Revision D.01), Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- F. Jensen, Introduction to Computational Chemistry, John Wiley & Sons Ltd, Chichester, UK, 2nd edn, 2007 Search PubMed.
- L. H. Mendoza-Huizar and C. H. Rios-Reyes, J. Mex. Chem. Soc., 2011, 3, 142–147 Search PubMed.
- S. Martinez, Mater. Chem. Phys., 2003, 77, 97–102 CrossRef CAS.
- R. G. Pearson, Inorg. Chem., 1988, 27, 734–740 CrossRef CAS.
- A. B. Anderson and S. Mehandru, Surf. Sci., 1984, 136, 398–418 CrossRef CAS.
- K. Khaled, J. Solid State Electrochem., 2009, 13, 1743–1756 CrossRef CAS.
- A. Arya and E. A. Carter, J. Chem. Phys., 2003, 118, 8982–8996 CrossRef CAS.
- A. Arya and E. A. Carter, Surf. Sci., 2004, 560, 103–120 CrossRef CAS.
- L. Guo, X. Ren, Y. Zhou, S. Xu, Y. Gong and S. Zhang, Arabian J. Chem., 2015 DOI:10.1016/j.arabjc.2015.01.005.
- X. Luo, S. Zhang and L. Guo, Int. J. Electrochem. Sci., 2014, 9, 7309–7324 CAS.
- A. Singh, Y. Lin, M. A. Quraishi, L. O. Olasunkanmi, O. E. Fayemi, Y. Sasikumar, B. Ramaganthan, I. Bahadur, I. B. Obot and A. S. Adekunle, Molecules, 2015, 20, 15122–15146 CrossRef CAS PubMed.
- J. Tan, L. Guo, T. Lv and S. Zhang, Int. J. Electrochem. Sci., 2015, 10, 823–837 CAS.
- S. W. Bunte and H. Sun, J. Phys. Chem. B, 2000, 104, 2477–2489 CrossRef CAS.
- G. Liu, S. Chen, M. Hongfang and X. Liu, J. Serb. Chem. Soc., 2007, 72, 475–484 CrossRef CAS.
- M. J. McQuaid, H. Sun and D. Rigby, J. Comput. Chem., 2004, 25, 61–71 CrossRef CAS PubMed.
- H. Sun, P. Ren and J. Fried, Comput. Theor. Polym. Sci., 1998, 8, 229–246 CrossRef CAS.
- J. Yang, Y. Ren, A.-m. Tian and H. Sun, J. Phys. Chem. B, 2000, 104, 4951–4957 CrossRef CAS.
- A. Fouda, A. Al-Sarawy and E. El-Katori, Desalination, 2006, 201, 1–13 CrossRef CAS.
- M. Morad and A. K. El-Dean, Corros. Sci., 2006, 48, 3398–3412 CrossRef CAS.
- Y. Yan, W. Li, L. Cai and B. Hou, Electrochim. Acta, 2008, 53, 5953–5960 CrossRef CAS.
- E. E. Stansbury and R. A. Buchanan, Fundamentals of Electrochemical Corrosion, ASM International, Materials Park, Ohio, USA, 2000, pp. 3–6 Search PubMed.
- P. Cao, R. Gu and Z. Tian, Langmuir, 2002, 18, 7609–7615 CrossRef CAS.
- C. Cao, Corros. Sci., 1996, 38, 2073–2082 CrossRef CAS.
- I. Lozano, E. Mazario, C. Olivares-Xometl, N. Likhanova and P. Herrasti, Mater. Chem. Phys., 2014, 147, 191–197 CrossRef CAS.
- F. S. de Souza and A. Spinelli, Corros. Sci., 2009, 51, 642–649 CrossRef CAS.
- S. A. Umoren, Y. Li and F. H. Wang, Corros. Sci., 2010, 52, 1777–1786 CrossRef CAS.
- A. A. Al-Amiery, A. A. H. Kadhum, A. B. Mohamad, A. Y. Musa and C. J. Li, Materials, 2013, 6, 5466–5477 CrossRef CAS.
- H. Cang, Z. Fei, W. Shi and Q. Xu, Int. J. Electrochem. Sci., 2012, 7, 10121–10131 CAS.
- L. Larabi, O. Benali, S. Mekelleche and Y. Harek, Appl. Surf. Sci., 2006, 253, 1371–1378 CrossRef CAS.
- J. Cruz, R. Martınez, J. Genesca and E. Garcıa-Ochoa, J. Electroanal. Chem., 2004, 566, 111–121 CrossRef CAS.
- G. Gece, Corros. Sci., 2008, 50, 2981–2992 CrossRef CAS.
- L. M. Rodríguez-Valdez, W. Villamisar, M. Casales, J. Gonzalez-Rodriguez, A. Martínez-Villafañe, L. Martinez and D. Glossman-Mitnik, Corros. Sci., 2006, 48, 4053–4064 CrossRef.
- W. Yang and W. J. Mortier, J. Am. Chem. Soc., 1986, 108, 5708–5711 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Mol. Graphics Modell., 2012, 38, 314–323 CrossRef CAS PubMed.
- S. T. Arab, Mater. Res. Bull., 2008, 43, 510–521 CrossRef CAS.
- K. Khaled, Appl. Surf. Sci., 2006, 252, 4120–4128 CrossRef CAS.
- K. Khaled, K. Babić-Samardžija and N. Hackerman, Electrochim. Acta, 2005, 50, 2515–2520 CrossRef CAS.
- Addinsoft, XLSTAT 2016.02, 2016, http://www.xlstat.com.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11373g |
| This journal is © The Royal Society of Chemistry 2016 |







