Rational design of plasmonic catalysts: matching the surface plasmon resonance with lamp emission spectra for improved performance in AgAu nanorings†
Abstract
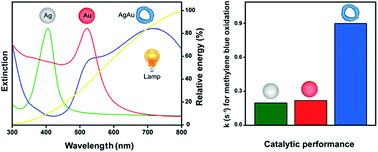
In order to enable practical applications of SPR-excitation in heterogeneous catalysis, facile procedures for the synthesis of plasmonic catalysts as well as the use of commercially available and inexpensive lamps as the excitation source are highly desirable. In this context, the development of catalysts displaying SPR extinction that matches, as much as possible, the emission spectra of commercially available lamps represent an intuitive strategy to maximize performance. We report the design and facile synthesis of AgAu nanorings displaying SPR extinction that closely matches the emission spectra of a commercial halogen–tungsten lamp. The AgAu nanorings were employed as catalysts for the SPR-mediated oxidation of methylene blue in the liquid phase (water as the solvent), under ambient conditions, and using a halogen–tungsten lamp as the only energy input. The activity of the nanorings was benchmarked against Ag and Au nanospheres. We found that the activity of the nanorings was higher relative to the nanospheres, and that both hot electrons and holes generated as a result of the SPR excitation participated in the methylene blue oxidation reaction with similar relative contributions. Our results show that the rational design of metallic nanostructures plays an important role for enabling practical applications in the field of plasmonic catalysis, in which facile procedures can be employed for the synthesis of the catalysts (attractive for large-scale production) and commercial lamps may be used as the only energy input.

 Please wait while we load your content...
Please wait while we load your content...