Boric acid functionalized ratiometric fluorescence probe for sensitive and on-site naked eye determination of dopamine based on two different kinds of quantum dots
Abstract
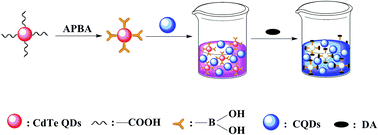
In this work, 3-aminophenylboronic acid (APBA) modified CdTe quantum dots (QDs) and carbon quantum dots (CQDs) were combined and used as a ratiometric fluorescence probe for the on-site naked eye detection of dopamine (DA). The probe combined the ratiometric fluorescence technique with boric acid functional materials together, and had the features of sensitivity and selectivity. Two kinds of QDs with different emission wavelengths were mixed into one system. To simplify the experimental operation processes, the blue-emission CQDs were introduced to serve as the reference signal, while the red-emission CdTe QDs acted as the response signal. With the addition of DA, the cis-diol compounds of DA covalently linked with APBA on the surface of the CdTe QDs, then the red-emission of the CdTe QDs decreased gradually and the blue-emission of the CQDs kept constant due to the surface quenching state induced mechanism, resulting in the color change of the mixture (from deep pink to blue). Under optimum measurement conditions, the proposed ratiometric probe had the advantage of sensitive detection of DA in a concentration range of 10–220 μM with a detection limit of 0.36 μM. The developed ratiometric fluorescence probe was proved to detect other cis-diol substances, like gallate and catechol. In addition, the developed probe was also applied for the detection of DA in human serum samples successfully. The present study provides a new and facile approach for the detection of DA and other similar substances without the requirement of complex equipment.

 Please wait while we load your content...
Please wait while we load your content...