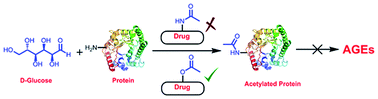
Pharmaceutical intervention for reduction of advanced glycation end products (AGEs) is considered as a therapeutic strategy to attenuate the pathogenesis of diabetes. Many molecules have been reported to possess antiglycation activity, one such example is acetylsalicylic acid (aspirin). It protects proteins from glycation by acetylating the lysine residues. Therefore, in this study we have synthesized and screened molecules containing free N-acetyl, O-acetyl and acetophenone groups. All the selected molecules in this study showed glycation inhibition but interestingly, only molecules with O-acetyl but not N-acetyl and acetophenone groups were capable of acetylating lysine residue. Furthermore, we have demonstrated that pre-acetylation or aspirin treatment prior to the induction of diabetes helps in reducing HbA1c and AGE formation in the streptozotocin induced diabetic mice. Hence pre-acetylation may have an additional therapeutic efficacy of reducing AGE levels in vivo. Incorporation of O-acetyl group into anti-diabetic molecules could be a useful strategy, as it may have an additive effect in reducing AGEs. Identification of such novel acetylating agents represents a new area in the drug discovery process.