Abuse tolerance behavior of layered oxide-based Li-ion battery during overcharge and over-discharge†
Kun Qiana,
Yiyang Lia,
Yan-Bing Hea,
Dongqing Liua,
Yong Zhengb,
Dan Luoa,
Baohua Li*a and
Feiyu Kanga
aEngineering Laboratory for the Next Generation Power and Energy Storage Batteries, Graduate School at Shenzhen, Tsinghua University, Shenzhen 518055, China. E-mail: libh@mail.sz.tsinghua.edu.cn
bSchool of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China
First published on 26th July 2016
Abstract
The slight abuse of lithium ion power batteries is inevitable during the practical charge/discharge process. Herein, we investigated the cycle decay behavior of Li(Ni1/3Co1/3Mn1/3)O2/graphite (NCM/C) high-power battery during slight overcharge (110% SOC) and over-discharge (2 V for lower cut-off voltage). The results show that the cycle life of NCM/C battery is about 1500 cycles at 45 °C, whereas the slight overcharge would markedly accelerate the capacity degradation and shorten the cycle life (only 500 cycles). In contrast, the slight over-discharge causes less damage (about 1300 cycles). A post-mortem study further reveals that the severe aging during overcharge can be mainly ascribed to the capacity loss of the layered oxide cathode material (NCM), followed by an inhomogeneous degradation in the anode. The ex situ XRD results show that the origin of the differing abuse tolerance during overcharge and over-discharge is due to the different crystal structure stability of NCM, which is more unstable in the delithiation state than that in the lithiation state, particularly at an excess delithiation condition corresponding to the overcharge state. Through HR-TEM, it is observed that the NCM suffers from irreversible phase transformation (8–10 nm rock salt phase at surface) even with a slight overcharge. This work provides effective guidance for how to design a voltage control strategy in a battery management system and avoid the capacity decay of NCM/C high-power battery during overcharge and over-discharge application.
1. Introduction
Lithium-ion batteries (LIBs) using layered oxides cathodes (Li(Ni1/3Co1/3Mn1/3)O2 (NCM)) are promising solutions for new-generation electric vehicles (EVs).1–5 However, the safety and lifespan of NCM-based batteries still hinder their applications in low-carbon EVs.1,6–9 To maintain their excellent cycle life and safety performance, LIBs must work at a specified potential range, and not beyond.10 The commercial NCM-based lithium-ion cells are normally charged to a fixed upper potential at 4.2 V and discharged to a lower potential at 2.8 V with a suitable current.11 The potential of a single cell or few-cells module is easily controlled under strict charging/discharging conditions. However, for numerous series-parallel connected cells in battery packs, although a complex battery management system (BMS) is used to monitor and control each cell, accurate control of their voltage is still facing large challenges.10 Overcharge and over-discharge of a single battery, as the common form of abuse, is inevitable when BMS is unable to precisely control the voltage of each battery. Actually, the degree of abuse and the module life always depend on the precision of the BMS. In order to develop high-precision BMS and extend the cycle life of a module, it is of great importance to take into account the impact of abuse on cycle life when designing the BMS. Thus, it is of considerable significance to investigate the abuse tolerance of batteries.To date, there are numerous articles associated with the effects of overcharge or over-discharge on the thermal and electrochemical stability of Li-ion batteries.12–16 Discharging cells to voltages less than 1.5 V could lead to anodic dissolution of the copper (Cu) current collector, which causes terrible damage to the structure and performance of the cells.13 Omar Samuel Mendoza-Hernandez et al. compared the thermal runaway behavior of LiCoO2 and LiMn2O4 using 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 Li-ion cells at different states of charge, including overcharge.7 The results showed that both cathode materials exhibited a thermal runaway behavior at overcharge; however, the LiCoO2-based cell was found to be more thermally unstable than the cell using LiMn2O4 when the materials in the cathode are highly delithiated. N. Sharma et al. found that after overcharging the commercial 18
650 Li-ion cells at different states of charge, including overcharge.7 The results showed that both cathode materials exhibited a thermal runaway behavior at overcharge; however, the LiCoO2-based cell was found to be more thermally unstable than the cell using LiMn2O4 when the materials in the cathode are highly delithiated. N. Sharma et al. found that after overcharging the commercial 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650-type Li-ion batteries to 4.6 V, the completely discharged state anode is composed of LiC12, LiC18, and graphite, which differs from a conventional discharge anode in which only graphite is found at the discharged state.17 M. Ouyang et al. recently reported the overcharge-induced capacity fading of LiyNi1/3Co1/3Mn1/3O2 and LiyMn2O4 composite cathode. The battery showed no obvious capacity degradation until it was overcharged to 120% state of charge (SOC), started to swell when overcharged to 140% SOC, and ruptured when overcharged to 150% SOC or more.14 QingFeng Yuan et al. investigated the overcharge failure of NCM-based full cells. They found that the cathode reacts violently with the electrolyte and the cell internal temperatures increased to more than 200 °C when overcharged after 180% SOC. Through post-mortem analysis, they found lithium plating during serious overcharge to be the major cause.18 Lingling Zhang et al. found that the capacity degradation of an over-discharged battery is caused mainly by the dissolution of the copper current collector and the deposition of Cu on the anode surface.19 Salim Erol et al. investigated the impedance response of LiCoO2/C batteries to overcharge and over-discharge. The results proved that impedance spectroscopy is quite sensitive to the condition of the Li-ion battery. The magnitude of the impedance increased tremendously when the battery was either overcharged or over-discharged.15
650-type Li-ion batteries to 4.6 V, the completely discharged state anode is composed of LiC12, LiC18, and graphite, which differs from a conventional discharge anode in which only graphite is found at the discharged state.17 M. Ouyang et al. recently reported the overcharge-induced capacity fading of LiyNi1/3Co1/3Mn1/3O2 and LiyMn2O4 composite cathode. The battery showed no obvious capacity degradation until it was overcharged to 120% state of charge (SOC), started to swell when overcharged to 140% SOC, and ruptured when overcharged to 150% SOC or more.14 QingFeng Yuan et al. investigated the overcharge failure of NCM-based full cells. They found that the cathode reacts violently with the electrolyte and the cell internal temperatures increased to more than 200 °C when overcharged after 180% SOC. Through post-mortem analysis, they found lithium plating during serious overcharge to be the major cause.18 Lingling Zhang et al. found that the capacity degradation of an over-discharged battery is caused mainly by the dissolution of the copper current collector and the deposition of Cu on the anode surface.19 Salim Erol et al. investigated the impedance response of LiCoO2/C batteries to overcharge and over-discharge. The results proved that impedance spectroscopy is quite sensitive to the condition of the Li-ion battery. The magnitude of the impedance increased tremendously when the battery was either overcharged or over-discharged.15
Previous studies always focused on the effects of severe abuse and their impairing mechanism.16,20 However, slight abuse occurs much more frequently but is not easy to detect in practice. It is indeed vital to study the effects of slight abuse on the performance of batteries for high-precision BMS and long-life battery system design. In this paper, we investigated the effects of slight overcharge (110% SOC) and over-discharge (2 V as lower cut-off voltage) on electrochemical performance of Li(Ni1/3Co1/3Mn1/3)/graphite (NCM/C) high-power batteries to identify the capacity decay mechanism. This work reveals the abuse tolerance behaviors and capacity decay mechanism of a NCM/C battery under overcharge and over-discharge conditions and gives guidance to develop high-precision BMS and long-life battery system.
2. Experimental
The study employed LiNi1/3Co1/3Mn1/3O2/graphite lithium-ion high-power pouch cells with a nominal capacity of 2.2 Ah and a nominal voltage of 3.65 V. The electrolyte is composed of 1 M LiPF6 salt dissolved in ethylene carbonate (EC)/dimethyl carbonate (DMC) (3/7 by volume) solution. Fig. S1 (in ESI†) shows an image of the cell and the specifications.Long-term cycling tests were carried out in an incubator at 45 °C. The tests included three types of procedures, namely, the normal cycling procedure, the overcharge cycling procedure and the over-discharge cycling procedure. The cells performed with a normal cycling procedure were conducted with standard charge/discharge cycles (1C-rate constant current charge to 4.2 V, followed by constant voltage charge at 4.2 V until the current decreased to less than 0.02C-rate, 1C-rate constant current discharge to 2.8 V with a 5 minute rest between charge and discharge). The overcharge cycling procedure was set with a 1C-rate current charge to 70% SOC and continued charging with a 0.1C-rate current to 110% SOC. Discharge was performed at a constant current of 1C-rate and terminated at the voltage limit of 2.8 V. The over-discharge cycling procedure was conducted the same as the standard charge/discharge cycles except that the terminated voltage of discharge was 2.0 V. The cells performed with the normal cycling procedure, overcharge cycling procedure and over-discharge cycling procedure are marked as NOR, OC and OD in the following discussion, respectively.
To establish the aging status and determine the abuse tolerance, reference performance tests (RPTs) were carried out at periodic intervals during long term cycling testing. The RPTs were conducted at 25 °C, including a static capacity test, a C/25-rate charge/discharge test and a direct current resistance (DCR) test. The static capacity test was conducted with a 1C-rate discharge to 2.8 V, followed by a standard charge/discharge to obtain the actual capacity. Moreover, through C/25-rate charge/discharge test, the relationship between the capacity and cell potential under quasi-equilibrium conditions could be acquired and the incremental capacity curves were obtained to investigate the aging process. Furthermore, a determination of DCR was conducted with a 10 s 4C-rate discharge pulse at 10% depth of discharge to evaluate the resistance degradation during cycling testing.
Electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) measurements were performed using an impedance analyser (Solartron 1470E; UK) at 25 °C in the frequency range from 100 kHz to 10 mHz. XRD measurements were conducted with a Rigaku D/max 2500/PC using CuKα radiation with λ = 1.5418 Å at a range of 10–80°. Half-cells (CR2032) tests were conducted by a battery test system (Land CT2001A; China) at 25 °C. The crystal structures of the NCM particles were examined using a 300 kV field emission transmission electron microscope (FE-TEM; Tecnai G2F30; FEI, Hillsboro, OR, USA). For HR-TEM analysis, the active material was racked out and dispersed in ethanol using a sonicator before being transferred onto a lacey carbon-supported Cu grid.
3. Results and discussion
3.1 The aging behaviors of NCM/C batteries under NOR, OD and OC tests
In the long-term cycling tests, the NOR, OD and OC cells showed differing decay behaviors. Specifically, the capacity of the OC cells decreased rapidly from 2.25 Ah to 1.8 Ah after 500 cycles (80% capacity retention, end of life, Fig. 1a). The capacity loss was much smaller in the NOR and OD cells (95% capacity retention) during the first 500 cycles as if there is no impairment from over-discharge. With cycling, over-discharge finally showed an accelerated aging effect on the capacity decay, shortening the cycle life from 1500 cycles at normal condition to 1300 cycles (Fig. 1b). However, the accelerating effect is not as intense as overcharge.To understand the differing abuse tolerances in NOR, OC and OD cells, incremental capacity (dQ/dV) measurements and impedance tests were performed on the cells. Incremental capacity is used widely to probe cell degradation over a cycle life test.21–23 By analysing the evolution of the dQ/dV peaks, the detailed capacity decay mechanism that the cell suffered can be determined. Fig. 2 displays the dQ/dV curves plotted with various cycles. For the cells with overcharge cycling procedure (Fig. 2c), the dQ/dV peaks show an obvious shift to lower voltages as well as the peak magnitude decrease with cycling. The decay of the dQ/dV peaks indicates the loss of NCM materials.24 The shift of the dQ/dV peaks may result from SEI growth, electrolyte oxidation or an increase in internal resistance.23,25 In contrast, the OD and NOR cells show less dQ/dV peak decay and dQ/dV peak shift compared to the OC cells, indicating that the NCM/C cell is of greater tolerance on over-discharge. The evolution of DCRs for the cells during the long-term cycling tests is displayed in Fig. 3a. Before cycling, the DCRs of the cells were similar at around 23 mΩ, illustrating the excellent consistency of the cells. After the first 250 cycles, the DCRs of the cells decreased to 17–18 mΩ, which may result from activation of the electrode material, including the initial SEI formation process. The SEI layers have very good ionic conduction. They are formed on the surface of the active material, enhancing internal contact between particles and electrolyte and providing a larger region for the electrochemical reaction. The observed decrease in bulk resistance (Rb) and charge transfer resistance (Rct) for NOR cells in the first 250 cycles (EIS data, Fig. S4†) well supports the electrode material activation process. With cycling, the DCRs of the OC cells increase to 22 mΩ, whereas that of the NOR and OD cells remain stable at around 17 mΩ, showing slight differences. The impedance behavior is in agreement with the cycle performance and IC analysis, proving that the NCM-based cell is sensitive to slight overcharge.
 | ||
| Fig. 2 Evolution of incremental capacity (IC) curves over various cycles for (a) NOR cell, (b) OD cell and (c) OC cell. | ||
 | ||
| Fig. 3 (a) DCR plotted as a function of the cycle number for the OC, OD and NOR tests (two cells for each test). (b) Nyquist plots of the NOR, OD and OC cells after 500 cycles. | ||
The electrochemical impedance spectra, powerful tool used to classify the potential reason for capacity decay, were also implemented on the NOR, OC and OD cells after 500 cycles. The spectra were simulated by Z-view software using an equivalent circuit (Fig. 3b). The bulk resistance (Rb) and charge-transfer resistance (Rct) were distinguished and listed in Fig. 3b. From EIS analysis, it can be seen that the total impedance (Rb + Rct) of the OC cell is twice as high as that of the NOR cell, drawing the same conclusion that the NCM-based cell is sensitive to overcharge. Furthermore, the aged OC cell obviously suffers from an impedance increase in both of Rb and Rct, indicating that overcharge not only induced severe degradation of particle to particle contact but also impaired the interfacial charge-transfer reaction process. For the OD cell, the total impedance was 1.4 times that of the NOR cell, indicating a considerable accelerated aging effect by over-discharge. In addition, Rb (for OC cell) increased by 3.79 times, whereas Rct was only 1.95 times that of the OC cell vs. OD cell. This indicates that the electrolyte degradation plays a very important role in the capacity decay of full cells. The EIS results show a clear comparison of resistance evolution for the OC and OD cells, which supplemented the capacity, IC, and DCR analysis on their differing aging behaviors. Based on these preliminary analyses, we conducted post-mortem analysis to give an exact analysis on the degradation mechanism for OC and OD cells in the next part.
3.2 Post-mortem analysis
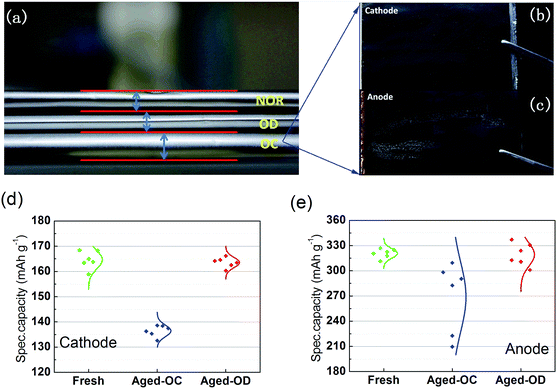
Fig. 4a shows the side faces of the cycled cells with 80% capacity retention. Obvious swelling was observed in the OC cell, illustrating that some undesired gassing reactions occurred with overcharge. To investigate the causes for the severe degradation of OC cell, the fresh cell and aged cell (OC) were disassembled in an argon atmosphere. Fig. 4b and c show the images of the disassembled electrodes. The surface of the OC-NCM cathode is clean and intact, whereas some upheaval and reaction products are observed on the OC-graphite anode, showing a dirty and rugged surface. This indicates that some side reactions took place at the interface of electrolyte and anode, which may induce the gas evolution and anode degradation.The disassembled graphite anodes and NCM cathodes were washed with dimethyl carbonate (DMC) and reassembled in coin-type half-cells. In this process, the small electrodes for half-cells were punched randomly from the electrodes of full cell. Six half-cells were made for each cathode and anode. To evaluate the capacity loss of active material precisely and avoid polarization, a very low current (C/25-rate) was carried out to charge and discharge the half-cells and examine the specific capacities. Fig. 4d and e show the specific capacities of the active materials before and after cycling (500 cycles). The specific capacity obtained from the six half-cells was in good consistency for the fresh, OC-NCM and OD-NCM. The average capacity of OC-NCM obviously decreased from 165 mA h g−1 to 136 mA h g−1. Interestingly, the specific capacity retention of OC-NCM was around 82.4%, which is very close to capacity retention of the full cell (80%), indicating that this is the one of the main reasons for the capacity loss of the full cells. For the anode, the OC-graphite tests show inhomogeneous degradation. The specific capacities of the six graphite half-cells have large differences (the specific capacity retention is in the range of 66–95%). The non-uniform decay in OC-graphite anode may be ascribed to the local side reactions, as inferred from the photograph shown in Fig. 4c. Furthermore, there was an obvious reduce in height of peak (003) in the XRD patterns of aged OC-cathode, indicating that the crystal structure of NCM was damaged to some extent (Fig. 5a). Slow-scan cyclic voltammetry also shows that the aged cathode experiences a decrease in peak current at the position of lithiation and delithiation, supporting capacity loss of the NCM material (Fig. S2†). While for the anode, there is no obvious difference in the XRD pattern of the graphite phase between the new and the aged one, the peak height for the trace phase of Li2CO3 increases for aged anode, which may be due to the side effect on the anode surface (Fig. 5b). Post-mortem analysis clearly demonstrates that the decay of full cell capacity (OC) is mainly due to the capacity loss of the NCM material, followed by the inhomogeneous decay of the anode and side reactions. NCM half-cells cycling tests, shown in ESI (Fig. S3†), also provide evidence that the NCM cathode is not stable with a higher cut-off voltage. Unlike the OC cells, both the OD-NCM and OD-graphite electrodes only experience subtle degradation. The capacity loss of the full cell (OD) is about 5% (Fig. 1b), whereas the specific capacity loss of the cathode and anode is only about 1%. Combined with earlier EIS analysis in the article, the electrolyte degradation can be considered the main reason for the decay of OD cells.
3.3 The origin of the tolerance difference during overcharge and over-discharge
As discussed above, the capacity loss of NCM was found to be one of the main reasons for the OC cells. In addition, XRD showed that there is considerable structural degradation for the NCM material. Thus, the stability of the NCM crystal structure is considered a potential origin of the tolerance difference of overcharge and over-discharge. To study the structural evolution of the NCM crystal under NOR, OC, and OD conditions, a series of XRD patterns was acquired by an ex situ XRD test with a different delithiated NCM material. During this process, the NCM material from the half-cells charged at various potentials were taken and ground before the measurement. The potential setup (vs. Li/Li+) covers the overcharge, over-discharge, and normal cycling conditions within the full cells (vs. graphite anode). In addition, we broadened the upper and lower voltage to give comprehensive analysis. By analysing the evolution process of the XRD patterns, the structural stability can be evaluated. The results of XRD measurements (Fig. 6) show that the reflections of the pristine NCM (red) are well indexed a hexagonal α-NaFeO2 lattice without any detectable impurities, which is in agreement with the previous report.5,26,27 In the α-NaFeO2 structure, Li-ions occupy the 3a site, whereas the transition metal ions (Mn, Co, Ni) and oxygen anions occupy the 3b and 6c sites, respectively.26,28,29 The oxygen sub lattice is a considered distortion in a hexagonal structure, leading to a separation of (006)/(102) and (108)/(110) peaks in the XRD patterns, which is usually used to evaluate the layered structure. The Li+/Ni2+ mixing may occur because the ionic radius is similar to each other and gives rise to a degradation of the electrochemical performance.2 The intensity ratio of the (003) and (104) peaks, which are characteristic of the Li+/Ni2+ mixing, is expected to be more than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 For our sample, the obvious splits of the (006)/(102) and (108)/(110) peaks with the high intensity ratio of (003) and (104) (I(003)/I(104) = 2.1) suggest that the NCM material possesses highly ordered hexagonal layered structure with low cation mixing.
2.5 For our sample, the obvious splits of the (006)/(102) and (108)/(110) peaks with the high intensity ratio of (003) and (104) (I(003)/I(104) = 2.1) suggest that the NCM material possesses highly ordered hexagonal layered structure with low cation mixing.
 | ||
| Fig. 6 Ex situ XRD patterns of the NCM material with different potentials vs. Li/Li+ under (a) normal range (b) overcharge state and (c) over-discharge state. | ||
With increasing potential from the open circuit potential (OCP) to 4.05 V (vs. Li/Li+), in Fig. 6a, the peaks of (003), (006) and (009) shifted to lower 2θ-angles, indicating an extension in the c axis, which could be due to the increasing electrostatic repulsion between the adjacent (Ni1/3Co1/3Mn1/3)O2 sheets accompanied by the extraction of lithium ions.30,31 Moreover, the shift of the (101) and (100) peaks to higher angles shows a contraction of the a and b axes in response to an increase in the average oxidation state of nickel and decrease in Ni–O average bond length.32 When lithium ions are extracted continually from the host material, the gliding occurs to release the stress, which may explain the inconspicuous shift of all the reflections from 4.05 V to 4.35 V.30
This ex situ XRD study during the normally cycled voltage range is consistent with the in situ XRD study on Li(Ni1/3Co1/3Mn1/3)O2 by Kyung-Wan Nam et al.,33 which proved the well reversibility of reflections during charge and discharge, indicating the good cyclability during specified voltage range, which was verified in cycling tests of half-cells. However, the ex situ XRD spectra of overcharged and over-discharged NCM cathode showed differences of structural evolution. For the overcharged NCM material, in Fig. 6b, an obvious decrease in intensity of all the reflections and the broadening peak width are observed with the increase in potential by every 0.1 V, indicating that some of the crystallinity is lost. Furthermore, additional peaks appear in the patterns of the sample at potential of 4.65 V (Fig. 6b, ‘+’), suggesting that a phase transformation occurs, which may result from the valence alternation of Co3+/Co4+.26 In contrast, for the over-discharged NCM cathode, as shown in Fig. 6c, we cannot find any obvious structural change from the XRD patterns until the potential was lowered to 1.45 V. The behaviors of crystal structure evolution in the upper and lower potential range may explain the different abuse tolerance of OC and OD cells. The increased structural instability even with a slight overcharge of the NCM cathode results in the severe degradation of the OC cell. In contrast, for the OD cell, the layers of (Ni1/3Co1/3Mn1/3)O2 are fully filled with lithium in the low-voltage state, which will slow the Li diffusion kinetics. A greater over potential is required to make up for the sluggish diffusion kinetics and drive more Li ions into the host material with the simultaneous shear of the oxygen planes.32 This effect may also reduce the cell life although it not as drastic as that of overcharge.
Furthermore, through high-resolution TEM (HR-TEM) and fast Fourier transformation (FFT) studies, we found the phase transformation zone on the primary particle of the aged NCM material (OC). The representative structural change is shown in Fig. 7. The bulk region remained in the rhombohedral phase, whereas a phase transformation occurred on the near-surface surface region where a rock salt crystal structure was presented. The phase transformation zone was localized mainly on the surface and the thickness was about 8–10 nm. The HR-TEM results verify that overcharge obviously results in structural decay of the NCM material.
 | ||
| Fig. 7 (a) TEM images of the aged NCM primary particle. (b) HR-TEM images and FFTs of the bulk and surface region. | ||
4. Conclusions
In summary, NCM/C high-power batteries present quite different abuse tolerance behaviors towards the slight overcharge and over-discharge process. Even slight overcharge would markedly accelerate the capacity degradation and shorten the cycle life of NCM/C batteries. For example, the cycle life decreased to 500 cycles even at a slightly overcharged cycle at 45 °C, which is much less than the normally cycled life of 1500 cycles. The batteries show much stronger abuse tolerance towards the over-discharge. The mechanism of severe capacity decay under overcharge condition was ascribed to the reversible capacity loss of the NCM material, followed by inhomogeneous decay of the anode and side reactions. The unstable crystal structure of NCM under the high-delithiation state would result in an irreversible phase transformation (8–10 nm rock salt phase at surface) and capacity loss, which has been observed by HR-TEM. Therefore, it is necessary to construct a modification layer to stabilize and protect the outmost surface of the NCM particles.Acknowledgements
This study was supported by the National Key Basic Research Program of China (2014CB932400), the National Natural Science Foundation of China (51202121 and 51232005), the Shenzhen Basic Research Project (No. ZDSYS20140509172959981 and JCYJ20140417115840246), and the Guangdong Province Innovation R&D Team Plan for Energy and Environmental Materials (2009010025).References
- N. Yabuuchi and T. Ohzuku, J. Power Sources, 2005, 146, 636–639 CrossRef CAS.
- E. Shinova, R. Stoyanova, E. Zhecheva, G. Ortiz, P. Lavela and J. Tirado, Solid State Ionics, 2008, 179, 2198–2208 CrossRef CAS.
- K. Amine, Z. Chen, Z. Zhang, J. Liu, W. Lu, Y. Qin, J. Lu, L. Curtis and Y.-K. Sun, J. Mater. Chem., 2011, 21, 17754 RSC.
- Y. Wei, J. Zheng, S. Cui, X. Song, Y. Su, W. Deng, Z. Wu, X. Wang, W. Wang, M. Rao, Y. Lin, C. Wang, K. Amine and F. Pan, J. Am. Chem. Soc., 2015, 137, 8364–8367 CrossRef CAS PubMed.
- K. M. Shaju, G. V. Subba Rao and B. V. R. Chowdari, Electrochim. Acta, 2002, 48, 145–151 CrossRef CAS.
- H. Konishi, T. Yuasa and M. Yoshikawa, J. Power Sources, 2011, 196, 6884–6888 CrossRef CAS.
- O. S. Mendoza-Hernandez, H. Ishikawa, Y. Nishikawa, Y. Maruyama and M. Umeda, J. Power Sources, 2015, 280, 499–504 CrossRef CAS.
- N. Yabuuchi, K. Yoshii, S. T. Myung, I. Nakai and S. Komaba, J. Am. Chem. Soc., 2011, 133, 4404–4419 CrossRef CAS PubMed.
- A. W. Golubkov, S. Scheikl, R. Planteu, G. Voitic, H. Wiltsche, C. Stangl, G. Fauler, A. Thaler and V. Hacker, RSC Adv., 2015, 5, 57171–57186 RSC.
- J. R. Dahn, J. Jiang, L. M. Moshurchak, M. D. Fleischauer, C. Buhrmester and L. J. Krause, J. Electrochem. Soc., 2005, 152, A1283 CrossRef CAS.
- B. R. Lee, H. J. Noh, S. T. Myung, K. Amine and Y. K. Sun, J. Electrochem. Soc., 2011, 158, A180 CrossRef CAS.
- J. Lamb, C. J. Orendorff, K. Amine, G. Krumdick, Z. Zhang, L. Zhang and A. S. Gozdz, J. Power Sources, 2014, 247, 1011–1017 CrossRef CAS.
- H. Maleki and J. N. Howard, J. Power Sources, 2006, 160, 1395–1402 CrossRef CAS.
- M. Ouyang, D. Ren, L. Lu, J. Li, X. Feng, X. Han and G. Liu, J. Power Sources, 2015, 279, 626–635 CrossRef CAS.
- S. Erol, M. E. Orazem and R. P. Muller, J. Power Sources, 2014, 270, 92–100 CrossRef CAS.
- Y. Zheng, K. Qian, D. Luo, Y. Li, Q. Lu, B. Li, Y.-B. He, X. Wang, J. Li and F. Kang, RSC Adv., 2016, 6, 30474–30483 RSC.
- N. Sharma and V. K. Peterson, J. Power Sources, 2013, 244, 695–701 CrossRef CAS.
- Q. Yuan, F. Zhao, W. Wang, Y. Zhao, Z. Liang and D. Yan, Electrochim. Acta, 2015, 178, 682–688 CrossRef CAS.
- L. Zhang, Y. Ma, X. Cheng, C. Du, T. Guan, Y. Cui, S. Sun, P. Zuo, Y. Gao and G. Yin, J. Power Sources, 2015, 293, 1006–1015 CrossRef CAS.
- N.-S. Choi, J.-G. Han, S.-Y. Ha, I. Park and C.-K. Back, RSC Adv., 2015, 5, 2732–2748 RSC.
- M. Dubarry, B. Y. Liaw, M.-S. Chen, S.-S. Chyan, K.-C. Han, W.-T. Sie and S.-H. Wu, J. Power Sources, 2011, 196, 3420–3425 CrossRef CAS.
- M. Dubarry, V. Svoboda, R. Hwu and B. Yann Liaw, Electrochem. Solid-State Lett., 2006, 9, A454 CrossRef CAS.
- S. Liu, L. Xiong and C. He, J. Power Sources, 2014, 261, 285–291 CrossRef CAS.
- A. J. Smith and J. R. Dahn, J. Electrochem. Soc., 2012, 159, A290–A293 CrossRef CAS.
- E. Zhao, Z. Hu, L. Xie, X. Chen, X. Xiao and X. Liu, RSC Adv., 2015, 5, 31238–31244 RSC.
- Y. Koyama, I. Tanaka, H. Adachi, Y. Makimura and T. Ohzuku, J. Power Sources, 2003, 119–121, 644–648 CrossRef CAS.
- J. L. Li, R. M. Yao and C. B. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 5075–5082 CAS.
- X. Bie, F. Du, Y. Wang, K. Zhu, H. Ehrenberg, K. Nikolowski, C. Wang, G. Chen and Y. Wei, Electrochim. Acta, 2013, 97, 357–363 CrossRef CAS.
- N. A. Chemova, M. Ma, J. Xiao, M. S. Whittingham, J. Breger and C. P. Grey, Chem. Mater., 2007, 19, 4682–4693 CrossRef.
- C.-K. Lin, Y. Ren, K. Amine, Y. Qin and Z. Chen, J. Power Sources, 2013, 230, 32–37 CrossRef CAS.
- Y. N. Zhou, J. Ma, E. Hu, X. Yu, L. Gu, K. W. Nam, L. Chen, Z. Wang and X. Q. Yang, Nat. Commun., 2014, 5, 5381 CrossRef PubMed.
- C. S. Johnson, J. S. Kim, A. J. Kropf, A. J. Kahaian, J. T. Vaughey, L. M. L. Fransson, K. Edstrom and M. M. Thackeray, Chem. Mater., 2003, 15, 2313–2322 CrossRef CAS.
- K.-W. Nam, W.-S. Yoon, H. Shin, K. Y. Chung, S. Choi and X.-Q. Yang, J. Power Sources, 2009, 192, 652–659 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11288a |
| This journal is © The Royal Society of Chemistry 2016 |