Understanding of nitrogen-doped carbon nanoparticles based solid phosphors for white light emitting diodes†
Hari Krishna Sadhanala,
Subrata Senapati and
K. K. Nanda*
Materials Research Centre, Indian Institute of Science, Bangalore-560012, India. E-mail: nanda@mrc.iisc.ernet.in; Tel: +91-80-22932449
First published on 11th July 2016
Abstract
The luminescence of carbon nanoparticles (CNPs) in solid form is believed to be quenched due to aggregation. Herein, we have reported the luminescence of N-doped CNPs (N-CNPs) in solution as well as in solid form. N-CNPs in solution exhibit excitation-dependent emission in solution and the luminescence intensity increases with nitrogen doping as well as with dilution. In the solid form, the luminescence is shown to be dependent on the power and follow a power dependency. The carrier recombination process are investigated in solid N-CNPs using a power dependent luminescence study. Finally, N-CNPs as a solid phosphor have been exploited for white light emission with CIE coordinates of (0.34, 0.34).
1 Introduction
Phosphors that exhibit both downconversion and upconversion with high efficiency have drawn considerable attention in a variety of applications such as phosphors in white-light-emitting diodes (WLEDs), light harvesters in solar cells, tracking materials in bioimaging, gas sensing, etc.1–10 One of the principles of WLEDs relies on wavelength conversion and a yellow phosphor is coated over a blue LED to generate white light. Ultraviolet (UV) light based WLEDs can also be achieved and semiconductor quantum dot (QD) based phosphors are being exploited. Unfortunately, QDs consist of toxic substances like lead or cadmium,11 and it is required to develop non toxic new phosphor materials for efficient WLEDs.Carbon nanoparticles (CNPs) are a new type of nanomaterials12 that emit visible light and the quantum yield (QY) is reported to be as high as 95% for N-doped CNPs (N-CNPs),13 which is comparable with best inorganic semiconductors.14 However, luminescence of N-CNPs is well-known to be quenched in the solid form which is believed to be due to aggregation,15,16 though report exists on the luminescence of CNPs in solid form also. Usually, the CNPs are embedded in a polymer matrix for the white light applications.
As luminescence of CNPs and N-CNPs in solid form is very much essential for light-emitting phosphors,17,18 it is, therefore, prime importance to investigate the luminescence in solid form and understand the possible origin of recombination process. In this letter, we report the luminescence of N-CNPs in solution as well as in solid form. Effect of N at% on luminescence QY has also been reported. The N-CNPs in solution exhibit intense bluish-white emission when exposed to UV light and the luminescence intensity increases with dilution. The QY increases with the N at% and is found to be comparable with broad-luminescent semiconductor nanostructures. One of the interesting observations is that N-CNPs also exhibit luminescence in the solid form that depends on the power of the excitation and sheds light on the recombination process. Finally, we have demonstrated the white light emission from the UV LED coated with N-CNPs embedded in poly vinyl pyrrolidine (PVP) polymer matrix.
2 Experimental section
2.1 Materials
Coffee powder was purchased from Dharshan Pvt and Millipore water was in all experiments.2.2 Synthesis
Herein, we adopted a hydrothermal method for the preparation of N-CNPs with different N at% (Fig. S1†) from the filter coffee powder by varying reaction time.19 Briefly, 0.8 g of filter coffee powder was mixed in 40 mL de-ionized water. The resultant mixture is then transferred into 50 mL Teflon-lined stainless steel autoclave and heated at 200 °C for different durations in a muffle furnace. After naturally cooling to room temperature, by using centrifugation, the settled black solid at the bottom of the Teflon flask was separated from the yellow solution of N-CNPs. The prepared N-CNPs at 3, 6, 9 and 12 h are referred as D3, D6, D9 and D12, respectively with different N at% (Table 1).| Sample | Time (h) | Diameter (nm) | C at% | O at% | N at% | N/C at% ratio | Quantum yield, ΦN-CNPs (%) |
|---|---|---|---|---|---|---|---|
| D3 | 3 | 2.8 | 75.5 | 20.5 | 4.0 | 4.9 | 4.6 |
| D6 | 6 | 3.2 | 73.5 | 21.7 | 4.8 | 5.4 | 4.9 |
| D9 | 9 | 3.9 | 72.8 | 22.2 | 5.0 | 6.0 | 6.6 |
| D12 | 12 | 4.2 | 68.7 | 24.5 | 6.8 | 8.0 | 13.7 |
For N-CNPs/PVP film preparation, 100 mg of PVP in 10 mL of N-CNPs solution is mixed followed by stirring for 30 min. Then the solution is dropcasted on UV LED and dried under incandescent lamp that leads to the formation of N-CNPs/PVP film.
2.3 Characterization
N-CNPs are characterized by Hitachi U-2900 UV-visible spectrophotometer. Horiba Jobin Yvon fluoromax-4 spectrofluorometer with Xe lamp is used for downconversion as well as upconversion PL measurements. For upconversion PL measurements; excitation wavelength range 950–1100 nm and emission wavelength range 450–700 nm. Optical characteristics such as color rendering index (CRI), Commission Internationale de l’Eclairage (CIE) chromaticity coordinates, correlated color temperature (CCT), and luminous efficacy were calculated using an integrating sphere (Avantes Avaspec-2048 spectrometer) under a forward bias of 30 mA (applied to UV LED). WITec system with 355 nm excitation laser is employed for the photoluminescence (PL) studies.2.4 Quantum yield (QY) calculation
For the estimation of QY, quinine sulphate (QS) is dissolved 0.05 M H2SO4 solution and as-prepared N-CNPs are diluted with water for optimum luminescence intensity. An UV-visible Hitachi 2900 absorption spectrometer is used to record absorption values of QS and N-CNPs, and Horiba Jobin Yvon fluoromax-4 spectrofluorometer is used to record PL at 365 nm excitation at room temperature. The QY of N-CNPs is calculated by using below equation| ΦN-CNPs = ΦQS(θN-CNPs/θQS)(ηQS/ηN-CNPs)(αQS/αN-CNPs), |
3 Results and discussion
To explore the optical properties, we performed the UV-visible spectroscopy studies on D3–D12 and we observed absorption bands between 200 and 350 nm (Fig. S2†), similar to GQDs.20 The peaks between 200 and 280 nm are due to π–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C while the peaks between 280 and 350 nm correspond to n–π* transition of C
C while the peaks between 280 and 350 nm correspond to n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups present at the edges. Due to incorporation of N atom in the carbon conjugated system with the increase of reaction time, peak at 280 nm (π–π* transition of C
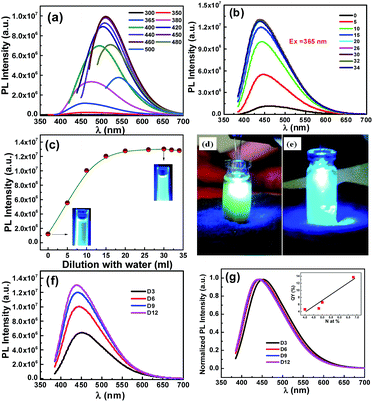
O functional groups present at the edges. Due to incorporation of N atom in the carbon conjugated system with the increase of reaction time, peak at 280 nm (π–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) is gradually decreasing. A detailed PL study of N-CNPs in solution has been carried out by using different excitation wavelengths at room temperature and the PL spectra of D12 are shown in Fig. 1a. It is evident that D12 (Fig. 1a) and D3–D9 (Fig. S3†) exhibit excitation-dependent luminescence which is due to different energy levels associated with various surface states18,20,21 as is the case with GQDs and N-GQDs.20 An increase in PL intensity (∼six times) is observed as the colloidal solution is diluted with water (Fig. 1b), which is due to minimization of the self-quenching of emitted light among N-CNPs.22 With further increase in dilution, the concentration of luminescence centers (N-CNPs) decrease, thereby the intensity decreases23 as shown in Fig. 1c. At a dilution of 30 mL, D12 exhibited bright cool white luminescence under 365 nm illumination as shown in the insets of Fig. 1c. Unlike similar size of 2–5 nm green luminescent GQDs24 and blue luminescent N-GQDs,25 our D12 emits intense bluish white luminescence. It may be noted here that blue luminescence is due to the intrinsic states, while the green luminescence is due to oxygeneous group26 and bluish white luminescence is obtained when exposed to UV light. Bright cool white light is also obtained when a UV light emitting diode (LED) is dipped in as-prepared as well as in diluted D12 as shown in Fig. 1d and e.
C) is gradually decreasing. A detailed PL study of N-CNPs in solution has been carried out by using different excitation wavelengths at room temperature and the PL spectra of D12 are shown in Fig. 1a. It is evident that D12 (Fig. 1a) and D3–D9 (Fig. S3†) exhibit excitation-dependent luminescence which is due to different energy levels associated with various surface states18,20,21 as is the case with GQDs and N-GQDs.20 An increase in PL intensity (∼six times) is observed as the colloidal solution is diluted with water (Fig. 1b), which is due to minimization of the self-quenching of emitted light among N-CNPs.22 With further increase in dilution, the concentration of luminescence centers (N-CNPs) decrease, thereby the intensity decreases23 as shown in Fig. 1c. At a dilution of 30 mL, D12 exhibited bright cool white luminescence under 365 nm illumination as shown in the insets of Fig. 1c. Unlike similar size of 2–5 nm green luminescent GQDs24 and blue luminescent N-GQDs,25 our D12 emits intense bluish white luminescence. It may be noted here that blue luminescence is due to the intrinsic states, while the green luminescence is due to oxygeneous group26 and bluish white luminescence is obtained when exposed to UV light. Bright cool white light is also obtained when a UV light emitting diode (LED) is dipped in as-prepared as well as in diluted D12 as shown in Fig. 1d and e.
For comparison of luminescence intensity for D3–D12, the solutions are diluted to obtain optimum intensity. As the particle size increases from D3 to D12, a red shift was expected. Interestingly, it is found that the PL intensity increases (Fig. 1f and g) and an emission peak blue-shifts from D3 to D12. Therefore, the enhancement in the intensity and the blue-shift are attributed to the increase in the N-doping concentration.27 We evaluate the QY by taking quinine sulfate as a reference. The QY is found to be 4.6, 4.9, 6.6, and 13.7% for D3–D12, respectively, which are higher than that reported for carbon quantum dots,28–30 but lower than N-GQDs prepared from citric acid and EDA28 and N-CNPs from glycine.30 Overall, the QY increases with N at% (inset of Fig. 1g) due to formation of new surface states labelled as N-states which trap the electrons thereby facilitating the high yield of radiative recombination,13 and is comparable to that for semiconductor nanostructures.31–38
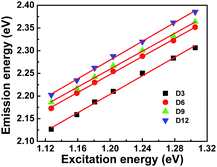
Now, we examine the upconversion PL as it is an important feature and it was observed frequently in CNPs and GQDs when excited by Xe lamp1,39 or lasers40–45 as excitation sources. In addition to normal PL, N-CNPs (D3–D12) exhibit excellent excitation-dependent upconversion, which is similar to downconversion behavior (Fig. S4a–d†). As shown in Fig. S4,† the upconverted emission peak of D3–D12 is red-shifted as the excitation wavelength changes from 950 to 1100 nm and is similar to CNPs.42,43 Interestingly, all the N-CNPs exhibit a linear relation between the energy of upconverted emission (Em) and excitation (Ex) as shown in Fig. 2. The fitting of the experimental data yields Em = 1.0Ex + δE with δE = ∼1.0 eV (Table 2). This clearly indicates that the energy δE difference between π and σ orbitals is ∼1.0 eV that is in excellent agreement with earlier reports.40,46,47
| Sample | Slope | Intercept (δE) | R2 |
|---|---|---|---|
| D3 | 1.01 | 0.98 | 0.9960 |
| D6 | 0.99 | 1.05 | 0.9985 |
| D9 | 0.98 | 1.07 | 0.9972 |
| D12 | 1.03 | 1.04 | 0.9980 |
It is well known that the luminescence of N-CNPs in solid form is quenched due to aggregation. Here, we have shown that luminescence depends on the laser power that sheds light on the carrier recombination mechanism. The PL spectrum of D12 (coated on a glass slide) with different laser power is shown in the Fig. 3a. As shown in the Fig. 3b, the variation of integrated PL intensity (I) with different laser power (P) follow a power law,48–50
| I ∼ Pα |
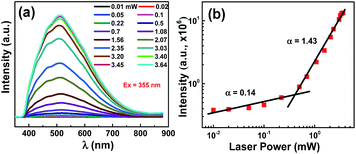 | ||
| Fig. 3 (a) PL spectra of N-CNPs with different laser power, (b) log–log plot of integrated PL intensity as function of laser power. The excitation of the laser is 355 nm. | ||
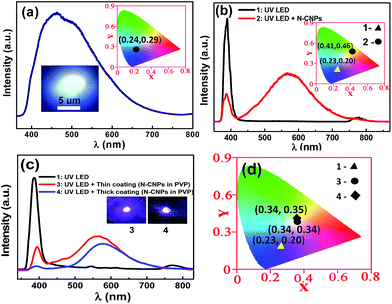
For practical applications, it is essential to evaluate the luminescence in solid state. In order to do so, N-CNPs drop-casted onto a glass slide and dried. The PL spectrum of the drop-casted film is shown in Fig. 4a and the optical photograph is shown in the inset. The optical photograph appears to be bright which clearly suggests that N-CNPs can be used as white light phosphors. Though the PL studies of the drop-casted film are carried out with 355 nm excitation, we choose UV LED that emits around 385 nm because of its availability. The emission spectra of UV LED and N-CNPs coated UV LED are shown in Fig. 4b. Similarly, the emission spectra of UV LED without and with N-CNPs and PVP film are shown in Fig. 4c. These results confirm that the solid film can convert UV light into visible light effectively and can be made suitable for white LED applications. The 1931 Commission Internationale l’Eclairage (CIE) diagram with coordinates for solid N-CNPs film (inset of Fig. 4a), UV LED and thin coating of N-CNPs on UV LED (inset of Fig. 4b) are (0.24, 0.29), (0.23, 0.20) and (0.41, 0.46), respectively. Interestingly, it was found that CIE coordinates for thin and thick coating of solid film of N-CNPs in PVP polymer on UV LED (Fig. 4d) are (0.34, 0.34) and (0.34, 0.35) which are close to that of pure white light (0.33, 0.33) and the corresponding cool white color is desirable for fluorescent lamps.51 Correlated color temperature (CCT), color rendering index (CRI) and luminous efficacy (LE) are found to be 5175 K, 84.7, and 39.5 lm W−1, respectively, for WLED (fabricated by coating N-CNPs/PVP on UV LED) with CIE color coordinate of (0.34, 0.34). Compared to the UV LED coated with mixture of N-CNPs and PVP, UV LED coated only with N-CNPs is displaying emission in the yellow region (inset of Fig. 4b) which is believed to be due to aggregation and strong reabsorption.
4 Conclusions
In summary, we have reported the luminescence of N-CNPs in solution as well as in solid form. The aqueous solution of N-CNPs exhibit excitation-dependent luminescence which is due to different energy levels associated with various surface states. Interestingly, bluish-white emission is observed when N-CNPs solution exposed to UV light and the intensity is enhanced with nitrogen concentration and dilution. The QY increases with the N at% and is found to be comparable to that for broad-luminescent semiconductor nanostructures reported in the literature. We show that the luminescence is due to the hole and donor recombination (h, D) at low power of excitation and the dominance of donor bound exciton emissions at high power. Finally, we demonstrate white light emission with CIE coordinates (0.34, 0.34) when an UV LED is coated with N-CNPs and PVP film.Acknowledgements
The authors acknowledge Department of Science and Technology (DST) and Defence Research and Development Organization (DRDO) for the financial support. The authors also acknowledge I.I.Sc for the financial assistance and Satish Laxman Shinde for his help in the PL studies of solid N-CNPs.Notes and references
- P. Roy, A. P. Periasamy, C. Chuang, Y.-R. Liou, Y.-F. Chen, J. Joly, C.-T. Liang and H.-T. Chang, New J. Chem., 2014, 38, 4946–4951 RSC.
- D. Chen and Y. Chen, Ceram. Int., 2014, 40, 15325–15329 CrossRef CAS.
- D. Chen, W. Xiang, X. Liang, J. Zhong, H. Yu, M. Ding, H. Lu and Z. Ji, J. Eur. Ceram. Soc., 2015, 35, 859–869 CrossRef CAS.
- D. Chen, L. Liu, P. Huang, M. Ding, J. Zhong and Z. Ji, J. Phys. Chem. Lett., 2015, 6, 2833–2840 CrossRef CAS PubMed.
- D. Chen and P. Huang, Dalton Trans., 2014, 43, 11299–11304 RSC.
- S. Zhuo, M. Shao and S.-T. Lee, ACS Nano, 2012, 6, 1059–1064 CrossRef CAS PubMed.
- G. Meng, Z. Chen, H. Tang, Y. Liu, L. Wei and Z. Wang, New J. Chem., 2015, 39, 9535–9542 RSC.
- J. Dwivedi, P. Kumar, G. Kedawat and B. K. Gupta, New J. Chem., 2015, 39, 5161–5170 RSC.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921–6939 RSC.
- Q. Liu, J. Peng, L. Sun and F. Li, ACS Nano, 2011, 5, 8040–8048 CrossRef CAS PubMed.
- J. Chen, D. Zhao, C. Li, F. Xu, W. Lei, L. Sun, A. Nathan and X. W. Sun, Sci. Rep., 2014, 4, 4085 Search PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- D. Qu, M. Zheng, L. Zhang, H. Zhao, Z. Xie, X. Jing, R. E. Haddad, H. Fan and Z. Sun, Sci. Rep., 2014, 4, 5294 CAS.
- E. Khon, S. Lambright, D. Khon, B. Smith, T. O'Connor, P. Moroz, M. Imboden, G. Diederich, C. Perez-Bolivar, P. Anzenbacher and M. Zamkov, Adv. Funct. Mater., 2012, 22, 3714–3722 CrossRef CAS.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. A. Tsang, X. Yang and S.-T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS PubMed.
- X. Li, Y. Liu, X. Song, H. Wang, H. Gu and H. Zeng, Angew. Chem., Int. Ed., 2015, 54, 1759–1764 CrossRef CAS PubMed.
- W. Kwon, S. Do, J. Lee, S. Hwang, J. K. Kim and S.-W. Rhee, Chem. Mater., 2013, 25, 1893–1899 CrossRef CAS.
- Y. Chen, M. Zheng, Y. Xiao, H. Dong, H. Zhang, J. Zhuang, H. Hu, B. Lei and Y. Liu, Adv. Mater., 2016, 28, 312–318 CrossRef CAS PubMed.
- H. K. Sadhanala, R. Nandan and K. K. Nanda, J. Mater. Chem. A, 2016, 4, 8860–8865 CAS.
- Q. Liu, B. Guo, Z. Rao, B. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS PubMed.
- M. Ghosh, S. K. Sonkar, M. Saxena and S. Sarkar, Small, 2011, 7, 3170–3177 CrossRef CAS PubMed.
- S. K. Sonkar, M. Ghosh, M. Roy, A. Begum and S. Sarkar, Mater. Express, 2012, 2, 105–114 CrossRef CAS.
- L. Tang, R. Ji, X. Li, K. S. Teng and S. P. Lau, J. Mater. Chem. C, 2013, 1, 4908–4915 RSC.
- H. K. Sadhanala, J. Khatei and K. K. Nanda, RSC Adv., 2014, 4, 11481–11485 RSC.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng, J. Hao and S. P. Lau, ACS Nano, 2012, 6, 5102–5110 CrossRef CAS PubMed.
- Z. Cheng, G. Tan, Y. Qiu, B. Guo, F. Cheng and H. Fan, J. Mater. Chem. C, 2015, 3, 6166–6171 RSC.
- C. Jiang, H. Wu, X. Song, X. Ma, J. Wang and M. Tan, Talanta, 2014, 127, 68–74 CrossRef CAS PubMed.
- J. J. Lander and J. Morrison, J. Appl. Phys., 1964, 35, 3593–3598 CrossRef CAS.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S.-Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- J. Jeong, M. Cho, Y. T. Lim, N. W. Song and B. H. Chung, Angew. Chem., Int. Ed., 2009, 48, 5296–5299 CrossRef CAS PubMed.
- S. Zhu, J. Zhang, C. Qiao, S. Tang, Y. Li, W. Yuan, B. Li, L. Tian, F. Liu, R. Hu, H. Gao, H. Wei, H. Zhang, H. Sun and B. Yang, Chem. Commun., 2011, 47, 6858–6860 RSC.
- X. Li, S. Zhang, S. A. Kulinich, Y. Liu and H. Zeng, Sci. Rep., 2014, 4, 4976 CAS.
- B. O. Dabbousi, J. Rodriguez-Viejo, F. V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen and M. G. Bawendi, J. Phys. Chem. B, 1997, 101, 9463–9475 CrossRef CAS.
- D. V. Talapin, A. L. Rogach, A. Kornowski, M. Haase and H. Weller, Nano Lett., 2001, 1, 207–211 CrossRef CAS.
- M. Molaei, E. S. Iranizad, M. Marandi and N. Taghavinia, AIP Adv., 2011, 1, 012113 CrossRef.
- M. A. White, A. L. Weaver, R. Beaulac and D. R. Gamelin, ACS Nano, 2011, 5, 4158–4168 CrossRef CAS PubMed.
- A. Hässelbarth, A. Eychmüller and H. Weller, Chem. Phys. Lett., 1993, 203, 271–276 CrossRef.
- L. E. Shea-Rohwer and J. E. Martin, J. Lumin., 2007, 127, 499–507 CrossRef CAS.
- L. E. Shea-Rohwer, J. E. Martin and D. F. Kelley, J. Electrochem. Soc., 2010, 157, J1–J7 CrossRef CAS.
- X. Jia, J. Li and E. Wang, Nanoscale, 2012, 4, 5572–5575 RSC.
- M. Li, W. Wu, W. Ren, H.-M. Cheng, N. Tang, W. Zhong and Y. Du, Appl. Phys. Lett., 2012, 101, 103107 CrossRef.
- C. Wang, X. Wu, X. Li, W. Wang, L. Wang, M. Gu and Q. Li, J. Mater. Chem., 2012, 22, 15522–15525 RSC.
- Y. Fang, S. Guo, D. Li, C. Zhu, W. Ren, S. Dong and E. Wang, ACS Nano, 2012, 6, 400–409 CrossRef CAS PubMed.
- A. Salinas-Castillo, M. Ariza-Avidad, C. Pritz, M. Camprubi-Robles, B. Fernandez, M. J. Ruedas-Rama, A. Megia-Fernandez, A. Lapresta-Fernandez, F. Santoyo-Gonzalez, A. Schrott-Fischer and L. F. Capitan-Vallvey, Chem. Commun., 2013, 49, 1103–1105 RSC.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S.-Y. Xie and Y.-P. Sun, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS PubMed.
- R. Hoffmann, J. Am. Chem. Soc., 1968, 90, 1475–1485 CrossRef CAS.
- D. Bourissou, O. Guerret, F. P. Gabbaï and G. Bertrand, Chem. Rev., 1999, 100, 39–92 CrossRef.
- V. A. Fonoberov, K. A. Alim, A. A. Balandin, F. Xiu and J. Liu, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 165317 CrossRef.
- T. Schmidt, K. Lischka and W. Zulehner, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 8989–8994 CrossRef.
- D. O. Dumcenco, Y. S. Huang, D. H. Kuo and K. K. Tiong, J. Lumin., 2012, 132, 1890–1895 CrossRef CAS.
- X. T. Feng, F. Zhang, Y. L. Wang, Y. Zhang, Y. Z. Yang and X. G. Liu, Appl. Phys. Lett., 2015, 107, 213102 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: More structural characterization, XRD, XPS, upconversion PL information. See DOI: 10.1039/c6ra11208k |
| This journal is © The Royal Society of Chemistry 2016 |