Vertical records of sedimentary PAHs and their freely dissolved fractions in porewater profiles from the northern bays of Taihu Lake, Eastern China†
Abstract
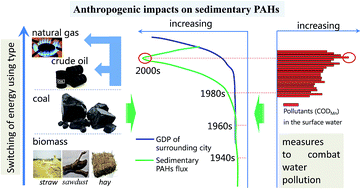
Sedimentary records of 16 priority PAHs in sediment cores collected from the northern bays of Taihu Lake were analyzed to evaluate the influence of anthropogenic impacts on the sedimentary PAHs. Freely dissolved PAHs in the overlying water/porewater matrices were also detected to help understand the toxicity of PAHs. High levels and ecological risk of PAHs were distributed in the northern bays, especially in Zhushan Bay. The concentrations, accumulation flux and total toxic benzo[a]pyrene equivalent (TEQcarc) of PAHs in sediment cores from the northern bays began to dramatically increase in the 1980s, and subsequently decreased after the 2000s. A significantly positive linear correlation (R2 > 0.90, p < 0.01) was observed between sedimentary flux and social development in the region during the period of the 1980s–2000s, while this relationship turned to a negatively exponential one (R2 > 0.99, p < 0.05) in the most recent 10 years. The decrease of PAHs flux may be attributed to the switch from coal to cleaner energy and good effectiveness of taking mandatory measures to combat water pollution, indicating that anthropogenic activities remarkably affected the load of sedimentary PAHs in the Lake. The freely dissolved PAHs were present in higher levels in porewater than those in overlying water; such a concentration gradient implies a potential flux of PAHs from porewater to overlying water. Taking the chronic toxicity values as reference, the hazard index (HI) values of all overlying water and porewater samples from the northern bay of Taihu Lake do not excess 1, indicating no or low ecological risk in water from the northern bay of Taihu Lake.

 Please wait while we load your content...
Please wait while we load your content...