Monitoring bicosomes containing antioxidants in normal and irradiated skin†
Abstract
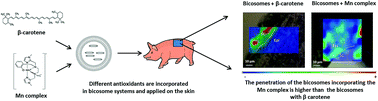
This study evaluates the penetration of bicosome systems incorporating two different antioxidants into normal skin and skin exposed to ultraviolet-visible radiation (UV-VIS) by Fourier-transform infrared microspectroscopy (FT-IR) using synchrotron radiation. Bicosomes are phospholipid assemblies based on mixtures of discoidal lipid structures protected by spherical lipid vesicles able to incorporate different molecules. In the current work, the antioxidants incorporated in these systems were β-carotene and a Mn complex as a superoxide dismutase (SOD) mimic. Additionally, a rhenium tri-carbonyl derivative was incorporated in the bicosome systems in order to map their penetration following the tag specific carbonyl signal by FT-IR microspectroscopy. The characterization of bicosome systems using the dynamic light scattering technique (DLS) showed a modification in the size of the systems containing β-carotene (Bcβ) or MnII complex (BcMn). After skin permeation, FT-IR results indicated a higher and deeper penetration of the BcMn system than the Bcβ system into the skin. Likely, the different physicochemical properties of both antioxidants could be responsible for this effect. Moreover, the penetration of both bicosome systems in irradiated skin was lower in comparison with the normal skin. This fact could be a consequence of the alteration of water transport in the skin during the irradiation process. In conclusion, these results indicated the effectiveness of bicosome systems as skin carriers, and provide information to protect skin under radiation using antioxidants.

 Please wait while we load your content...
Please wait while we load your content...