DOI:
10.1039/C6RA11132G
(Paper)
RSC Adv., 2016,
6, 59737-59748
Honeycomb-like CuO/ZnO hybrid nanocatalysts prepared from solid waste generated in the organosilane industry†
Received
29th April 2016
, Accepted 15th June 2016
First published on 15th June 2016
Abstract
We report the preparation of honeycomb-like CuO/ZnO (CZx/y) nanocatalysts with CuO nanospheres (NSs) adhered with ZnO nanoparticles (NPs) for the Rochow reaction. The synthesis was carried out via adsorption of Cu2+/Zn2+ ions on carbon black (CB) which acted as both the agglomeration inhibitor and the hard template, and followed by calcination in air. The low cost Cu2+/Zn2+ ions were recovered from the solid waste generated in the organosilane industry via a simple ammonia leaching treatment. The samples were characterized by X-ray diffraction, transmission electron microscopy, scanning electron microscopy, X-ray photoelectron spectroscopy, and temperature-programmed reduction. The as-obtained CZx/y nanohybrids had a honeycomb-like structure with large voids and openings among the CuO NSs. When re-used as a Cu-based catalyst for the Rochow reaction, the CZx/y NPs sample with an optimized ratio showed significantly improved dimethyldichlorosilane (M2) selectivity and silicon (Si) conversion as compared with the CuO/ZnO NPs prepared in the absence of CB, discrete CuO or ZnO NPs and the CuO/ZnO NPs with different compositions, mainly due to the unique honeycomb-like structure, smaller crystal size and synergistic electronic effect at the interface between Cu and ZnO in CZx/y NPs.
1. Introduction
Owing to structural features such as p–n heterojunctions, CuO/ZnO materials are promising in a wide range of applications,1–4 including chemical and humidity sensing,5,6 CO2 photoreduction,7 self-cleaning sensors8 and selective H2S detection.9–11 More recently, various nanoscale CuO/ZnO heterostructures, such as CuO/ZnO nanoflakes,12,13 CuO/ZnO core/shell heterostructured nanowires,14 heterojunction CuO@ZnO composites,15,16 flower-like CuO/ZnO heterostructured nanowires,17 corn-like CuO/ZnO structures18 and CuO/ZnO nanocorals,19 have been reported with improved catalytic performance as compared with their bulk counterparts. The methods used to prepare these materials mainly include chemical vapor deposition,20,21 chemical bath deposition22,23 and hydrothermal techniques.24–26 However, these methods often suffer from harsh reaction conditions, high cost, tedious synthesis processes and/or complicated equipment, and low productivity, thus hindering their eventual industrial application. To date, there is still a lack of a very cost-effective approach to the production of hierarchically structured CuO/ZnO, particularly at large scale.
Over the past few decades, the Rochow reaction has attracted intensive attention as it is the most economical route to produce methylchlorosilanes (MCSs) monomers in the organosilane industry.27–29 In this reaction, gaseous methyl chloride (MeCl) reacts with silicon (Si) with the assistance of catalysts, and the reaction products are shown in Scheme 1. Among these products, dimethyldichlorosilane ((CH3)2SiCl2, M2) is the most valuable one because it is a versatile building block for some important silicon rubbers and silicon oil. Hence, the selective synthesis of M2 is highly desired. It is reported that copper-based catalysts added with zinc-based promoters are effective in improving M2 selectivity and yield.30 In this reaction, due to the reaction equilibrium limitation, part of copper and zinc compounds as well as a large amount of solid Si particles will be inevitably remained, which are the so-called waste contact masses. In recent years, due to the rapid growth in production of the organic silicon monomer, large quantities of such kind of waste contact masses have been produced, and of course, many efforts have also been devoted to recovering valuable metals from waste contact masses and to exploring their application.31–33 The employed approaches for the recovery of such copper compounds generally contain two complicated processing steps: acid dissolution and precipitation with alkaline solution.34 Furthermore, the obtained particles are usually dense in most cases, generating unfavorable properties and difficulty in their utilization.
 |
| | Scheme 1 Main products generated in the Rochow reaction. | |
In this work, we report an efficient and sustainable route to recover Cu2+/Zn2+ ions from the aforementioned waste contact masses via a simple ammonia leaching treatment, and further prepare the honeycomb-like CuO/ZnO (CZx/y) nanohybrids with CuO nanospheres (NSs) connected to ZnO nanoparticles (NPs). In the synthesis, the Cu2+/Zn2+ ions were adsorbed on carbon black (CB) which acted as both the hard template and the agglomeration inhibitor, and followed with calcination in air. Comparing with the existing methods used, this process avoids use of any complicated equipment and expensive reagents, and can be readily scaled up. Furthermore, owing to the unique structure, smaller crystal size and synergistic electronic effect at the interface between Cu and ZnO, the as-prepared CZ80/20 NPs showed much higher M2 selectivity and Si conversion in the Rochow reaction as compared with the CZx/y NPs prepared in the absence of CB, the discrete CuO or ZnO NPs as well as the CZx/y NPs with different compositions. This work paves a new environmentally friendly and low-cost way for preparation of efficient CuO/ZnO catalysts via recovery of spent catalysts in the Rochow reaction.
2. Experimental
2.1 Sample preparation
All of the chemical reagents were purchased and used as received without further purification. Distilled water was used in all the experiments. The waste contact mass was provided by Tangshan City Sanyou Silicon Industry Co., Ltd (the molar ratio of Cu to Zn is 99 to 1 analyzed by ICP-OES). The preparation process of CuO/ZnO NPs is illustrated in Scheme 2. Firstly, 80 g of waste contact mass was dispersed in 400 mL of ethanol–water solution (volume ratio of ethanol to water was 1 to 3) under stirring to form a homogeneous suspension. Then, a mixture containing 40 mL of ammonia solution (28–30 wt%) and 11.2 g of ammonium carbonate (CH3COONH4) was slowly added, followed with vigorous stirring for 5 h at 50 °C (the so-called ammoniacal leaching). The resulting mixture was filtered to remove the silicon and other impurities, and the obtained copper and zinc ammonia precursor solution is named as A. Meanwhile, a given amount of zinc ammonia solution was also introduced into A to adjust the mole ratio of Cu2+ and Zn2+. Afterwards, certain amount of carbon black (CB, ranging from 0 g to 32 g) was added and the above mixture was continuously stirred for another 6 h to achieve sufficient adsorption of both Cu2+ and Zn2+ to form Cu2+/Zn2+/CB. Finally, the slurry was dried at 120 °C overnight and further calcined in air at 500 °C for 5 h to remove the CB. At the same time, the recovered ammonium hydroxide (NH4OH) can be reused in another round of ammoniacal leaching. The CuO/ZnO samples thus prepared are denoted as CZx/y, where x represents CuO mass fraction (100 × mCuO/m(CuO+ZnO)) and y represents ZnO mass fraction (100 × mZnO/m(CuO+ZnO)). The amount of added CB corresponds to 0–67 wt% relative to the theoretical sum weight of CuO and ZnO (100 × mCB/(m(CuO+ZnO) + mCB)). For the control experiments, the pure CuO or ZnO catalyst was prepared followed the above procedure by directly using Cu(CH3COO)2·H2O or Zn(CH3COO)2·H2O as the precursor in the presence of 67 wt% CB. The samples are denoted as CZ100/0 and CZ0/100 respectively.
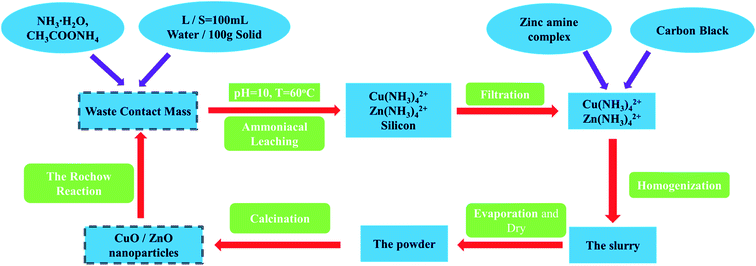 |
| | Scheme 2 The preparation process of honeycomb-like CZx/y materials. | |
2.2 Materials characterization
X-ray diffraction (XRD) analysis was performed on a PANalytical X'Pert PRO MPD using the Kα radiation of Cu (λ = 1.5418 Å). The crystallite size was calculated based on predominant crystal orientation using the Debye–Scherrer equation, in which the shape factor of K is 0.90. Moreover, the 2θ value of instrumental broadening is 0.06, which was subtracted in the test process. The microscopic feature of the samples was observed by field-emission scanning electron microscopy (SEM) (JSM-7001F, JEOL, Tokyo, Japan) with energy-dispersive spectroscopy (EDS) (INCA X-MAX, JEOL, Oxford, England) and transmission electron microscopy (TEM) (JEM-2010F, JEOL, Tokyo, Japan). H2-temperature programmed reduction (H2-TPR) measurements were carried out on an automated chemisorption analyzer (ChemBET pulsar TPR/TPD, Quantachrome). Upon loading of about 0.05 g of sample into a quartz U-tube, the sample was degassed at 150 °C for 30 min under helium. When the temperature was dropped to 30 °C, the gas was changed to 9.9% H2/He. Finally, the sample was heated from 30 to 900 °C at 10 °C min−1 under the H2/He flow (30 mL min−1). X-ray photoelectron spectroscopy (XPS) analysis was carried out on an ESCALAB 250Xi from Thermo Scientific Corporation using AlKα X-ray radiation. Stacking density of the samples was analyzed by Scott volumeter method (ISO 3923/2) on a BT-101 from Bettersize Instruments LTD. The elemental analysis was carried out with inductively coupled plasma optical emission spectrometer (ICP-OES) (Optima 5300DV, Perkin Elmer, American). Thermal gravimetric (TG) analysis was carried out on an EXSTAR TG/DTA 6300 (Seiko Instruments, Japan) at a heating rate of 2 °C min−1 in air.
2.3 Catalytic measurement
The catalyst evaluation was carried out on a typical lab-scale fixed-bed reactor.35 Silicon powder (20–50 mesh) and the prepared catalysts at a weight ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were mixed homogeneously to form a contact mass, which was loaded in the glass reactor. The reactor system was purged with purified N2 for 0.5 h followed by heating to 295–355 °C within 1 h under a N2 flow rate of 25 mL min−1. Subsequently, N2 was turned off, and CH3Cl gas with a flow rate of 25 mL min−1 was introduced into the reactor to react with Si followed by heating to 325 °C. After a given period of 24 h, the reaction was stopped. The gas product was cooled to liquid phase with the circulator bath controlled at −5 °C by a programmable thermal circulator (GDH series, Ningbo xinzhi biological technology Co., LTD). The products in the liquid solution were quantitatively analyzed on an Agilent Technologies (GC-7890A) gas chromatograph equipped with KB-201 column (60 m) and TCD detector. Gas chromatography-mass spectrometer system (GC-MS) was used for identification of the products, which were mainly comprised of methyltrichlorosilane (CH3SiCl3, M1), dimethyldichlorosilane ((CH3)2SiCl2, M2), trimethylchlorosilane ((CH3)3SiCl, M3), methyldichlorosilane (CH3SiHCl2, M1H), dimethylchlorosilane ((CH3)2SiHCl, M2H), low boiler (LB) and high boiler (HB). The selectivity of the products was calculated by the peak area ratio (in percentage). The spent contact masses (residual solid after reaction) containing unreacted Si powder and CZx/y compounds were weighed to obtain the ratio of weight difference of contact mass (before and after reaction) and weight of Si (before reaction) for the purpose of calculating the Si conversion (formula (1)).
1 were mixed homogeneously to form a contact mass, which was loaded in the glass reactor. The reactor system was purged with purified N2 for 0.5 h followed by heating to 295–355 °C within 1 h under a N2 flow rate of 25 mL min−1. Subsequently, N2 was turned off, and CH3Cl gas with a flow rate of 25 mL min−1 was introduced into the reactor to react with Si followed by heating to 325 °C. After a given period of 24 h, the reaction was stopped. The gas product was cooled to liquid phase with the circulator bath controlled at −5 °C by a programmable thermal circulator (GDH series, Ningbo xinzhi biological technology Co., LTD). The products in the liquid solution were quantitatively analyzed on an Agilent Technologies (GC-7890A) gas chromatograph equipped with KB-201 column (60 m) and TCD detector. Gas chromatography-mass spectrometer system (GC-MS) was used for identification of the products, which were mainly comprised of methyltrichlorosilane (CH3SiCl3, M1), dimethyldichlorosilane ((CH3)2SiCl2, M2), trimethylchlorosilane ((CH3)3SiCl, M3), methyldichlorosilane (CH3SiHCl2, M1H), dimethylchlorosilane ((CH3)2SiHCl, M2H), low boiler (LB) and high boiler (HB). The selectivity of the products was calculated by the peak area ratio (in percentage). The spent contact masses (residual solid after reaction) containing unreacted Si powder and CZx/y compounds were weighed to obtain the ratio of weight difference of contact mass (before and after reaction) and weight of Si (before reaction) for the purpose of calculating the Si conversion (formula (1)).| |
 | (1) |
3. Results and discussion
3.1 Preparation and characterization of CZx/y catalysts
Fig. 1 shows the X-ray diffraction (XRD) patterns of the as-synthesized CZ95/5, CZ80/20, and CZ50/50 samples with different CB loadings from 0 to 67 wt%. The diffractions observed at 2θ values of 32.5, 35.5, 39.0, 48.8, 53.3, 58.2, 61.5, 66.3, 68.0, 72.3 and 75.2° are attributed to (110), (−111), (200), (−202), (020), (202), (−113), (−311), (220), (311) and (−222) planes of CuO, respectively (JCPDS no. 05-0661), while the ones at 31.8, 34.4, 36.3, 47.5 and 56.6° correspond to the lattice planes of (100), (002) (101), (102) and (110) of ZnO (JCPDS no.070-2551), revealing that all the samples are composed of both CuO and ZnO phases. No other obvious crystalline impurities can be observed in these samples. The sharp peaks in all the XRD patterns indicate that all the samples have good crystallinity. Also, the diffraction intensity decreases progressively with the increase of the CB amount from 0 to 67%, suggesting the crystal size becomes smaller gradually.36 The average crystallite size calculated by the Scherrer formula based on the peak at 38.7° for CuO and 36.5° for ZnO are summarized in Table 1, together with the stacking density given by Scott volumeter method. It is found that with the increase of the Zn atomic percentage, the particle sizes of both CuO and ZnO are continuously increased. In contrast, with the increase of the CB amount, both the average crystallite diameters and the stacking density are decreased for all the samples. These results show that the amount of CB has a significant effect on the crystal size and structure of the CZx/y samples.
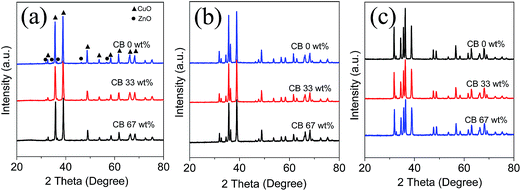 |
| | Fig. 1 XRD patterns of the prepared samples with different amounts of CB: CZ95/5 (a); CZ80/20 (b); CZ50/50 (c). | |
Table 1 The physicochemical properties of all the samples
| Sample |
CB amount (wt%) |
DCuOa (nm) |
DZnOa (nm) |
Stacking densityb (g cm−3) |
ICP (wt%) |
TMc (°C) |
| Cu |
Zn |
| The grain size of the prepared samples calculated from the XRD patterns, standard errors: 0.5 nm. Given by Scott volumeter method. Temperatures at the peak maximum from H2-TPR curves. |
| CZ95/5 |
0 |
31.3 |
33.4 |
1.53 |
0.95 |
0.05 |
— |
| 33 |
27.1 |
28.9 |
0.96 |
0.94 |
0.06 |
— |
| 67 |
26.7 |
28.7 |
0.61 |
0.95 |
0.05 |
302.7 |
| CZ80/20 |
0 |
31.9 |
34.1 |
1.66 |
0.81 |
0.19 |
— |
| 33 |
28.2 |
29.2 |
1.01 |
0.80 |
0.20 |
— |
| 67 |
28.1 |
28.4 |
0.64 |
0.81 |
0.19 |
291.5 |
| CZ50/50 |
0 |
33.5 |
39.7 |
1.87 |
0.50 |
0.50 |
— |
| 33 |
30.7 |
33.2 |
1.24 |
0.53 |
0.48 |
— |
| 67 |
29.5 |
31.7 |
0.82 |
0.52 |
0.48 |
259.4 |
The scanning electron microscopy (SEM) micrographs of the used waste contact mass and CB as well as various CZx/y catalysts prepared with different amounts of CB are shown in Fig. S1 and S2 in ESI† and in Fig. 2. The waste contact mass (Fig. S1†) is composed of the particles with irregular shape and a diameter of about 50 μm, of which the rough area is mainly the copper-based catalyst while the smooth area is the unreacted Si. CB with a particle size of about 40 nm shows an aggregated network structure (Fig. S2†). As clearly indicated in Fig. 2a, for CZ95/5 sample, the crystals prepared in the absence of CB comprise of densely and irregularly large blocks in the size range of 1–3 μm which are made up by primary particles less than 50 nm in size, indicating occurrence of extensive agglomeration. However, after the addition of a given amount of CB (33 wt%) in the preparation, the extent of agglomeration is reduced obviously, and the size of the obtained particles becomes smaller, ranging from 0.5–1 μm (Fig. 2b). Upon a closer observation to the magnified image, it is found that a large number of smaller particles (below 500 nm) are coated on the surface of these particles. When the added CB amount reaches 67 wt% (Fig. 2c), the particle size is further decreased to about 100–200 nm. Also, there are many voids among the particles, leading to the formation of a honeycomb-like nanoporous network structure consisting of nanospheres. In the cases of CZ80/20 and CZ50/50 (Fig. 2d–f and g–i), similar phenomenon is observed with CB weight ratio varying from 0 to 67%. These results clearly suggest that CB template is effective in preventing agglomeration of the primary particles during the crystallization process. The control experiments were carried out to obtain the individual CuO NPs and ZnO NPs. Both of them also exhibit honeycomb-like structure. It is noticed that the size of the CuO NPs (100–200 nm) (Fig. 2j) is similar to that of the CuO NPs in the CZx/y hybrid nanostructures, while the size of the ZnO NPs (400 nm) (Fig. 2k) becomes obviously larger compared to the ZnO NPs in the CZx/y hybrid nanostructures, suggesting that the presence of the Cu species affects the growth kinetics of ZnO NPs. A similar phenomenon has also been reported in literature.37–39 Furthermore, we observed that the morphology of the final product is related to the kind of Cu precursor. For Cu(NH3)4NO3, the obtained particles agglomerate into 2–3 μm dense blocks (Fig. S3†), while Cu(NH3)4CO3 precursor generates elongated shape with size of about 1 μm (Fig. S4†).
 |
| | Fig. 2 SEM micrographs of the prepared samples with different amounts of CB: CZ95/5 (a–c) (0 wt%, 33 wt% and 67 wt%); CZ80/20 (d–f) (0 wt%, 33 wt% and 67 wt%); CZ50/50 (g–i) (0 wt%, 33 wt% and 67 wt%); CZ100/0 (j) (67 wt%); CZ0/100 (k) (67 wt%). The inserts are corresponding magnified images. | |
Fig. 3 shows the transmission electron microscopy (TEM) images of the obtained products. As demonstrated in Fig. 3a, the as-prepared CZ95/5 sample without CB is composed of large blocks, consistent with the above SEM results (Fig. 2a). In contrast, the as-obtained sample in the presence of 67 wt% CB contains large sphere-like nanostructures with small NPs attached to their surface (Fig. 3b). The big NPs have a size distribution of 100–200 nm and are randomly distributed. The small peripheral NPs with size of 50–100 nm are distributed on the surface of the big NPs. Similarly, for both CZ80/20 and CZ50/50 samples, the same trend is observed after addition of CB (Fig. 3c–f). The representative magnified high-resolution transmission electron microscope (HRTEM) images of the CZ80/20 samples prepared with 67 wt% CB are shown in Fig. 3h and i. The clear lattice fringes have d-spacing of about 0.25 nm and 0.28 nm, corresponding to the (002) plane of monoclinic CuO and the (100) plane of hexagonal ZnO, respectively. Moreover, the interfacial regions between CuO and ZnO are clearly observed (Fig. 3g). The energy dispersive spectroscopy (EDS) analysis result for the CZ80/20 sample is shown in Fig. 4b, which reveals the existence of Cu, Zn and O elements. Additionally, the corresponding elemental mapping images (Fig. 4c and d) show the elemental distribution over large areas, confirming the homogeneous distribution of Cu and Zn elements. Finally, the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn weight ratios determined by ICP-OES verify that the compositions of the final products are close to the initial feeding ratio of the components, as shown in Table 1.
Zn weight ratios determined by ICP-OES verify that the compositions of the final products are close to the initial feeding ratio of the components, as shown in Table 1.
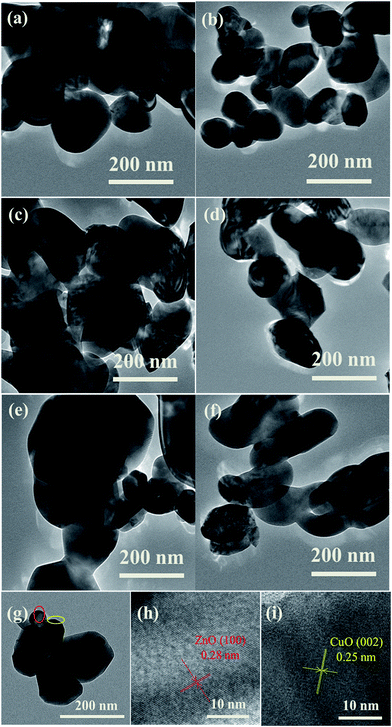 |
| | Fig. 3 TEM images of the prepared samples with different amounts of CB: CZ95/5 (a and b) (0 wt% and 67 wt%); CZ80/20 (c and d) (0 wt% and 67 wt%); CZ50/50 (e and f) (0 wt% and 67 wt%); and the corresponding HRTEM of CZ80/20 prepared with 67 wt% of CB (h and i) derived from (g) image. | |
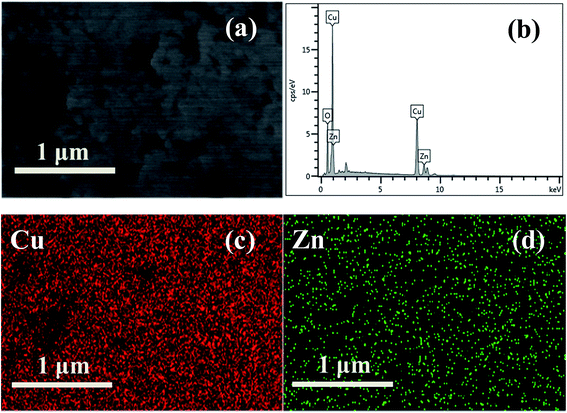 |
| | Fig. 4 SEM image (a), SEM-EDX spectra (b) and elemental mapping images of CZ80/20 with 67 wt% amount of CB (c and d). | |
The measured TG and DTA curves of the CZ80/20 precursor prepared with 67 wt% CB before calcination are shown in Fig. 5a. The DTA curve has three weight-loss peaks at different temperatures. The weight loss below 240 °C is attributed to the thermal decomposition of [Zn(NH3)4]2+ species, whereas the one in the temperature range of 250–350 °C to the decomposition of [Cu(NH3)4]2+ species, and for the one in the temperature range of 350–400 °C, it corresponds to the oxidation of CB.40 As demonstrated in Fig. 5b, the CB in the as-obtained CZ80/20 sample is removed completely after calcination at 500 °C in air (100 mL min−1) for 5 h, because there is no any weight loss in the range between 25 and 1000 °C. This is essential for further investigation of the catalytic properties.
 |
| | Fig. 5 TG and DTA curves of CZ80/20 precursor with 67 wt% CB (a) and CZ80/20 with 67 wt% CB (b). | |
3.2 Formation process of CZx/y NPs
Based on the above-mentioned XRD, SEM, TEM and TG analyses, a formation mechanism for the honeycomb-like CZx/y NPs is thus proposed, which is illustrated schematically in Fig. 6. First of all, upon the addition of the mixed solution of ammonium carbonate and ammonia, (Cu(NH3)4)2+ and (Zn(NH3)4)2+ ions are generated via ammoniacal leaching. During this stage, ammonia is employed as the complexation reagent to neutralize the leachate of the waste contact mass. Meanwhile, ammonium carbonate or ammonium bicarbonate can adjust the solution pH, preventing conversion of both Cu and Zn compounds into their respective hydroxides. In addition, NH4+ supplied by the ammonium salt can enhance the amine complexing reaction by removing OH−.
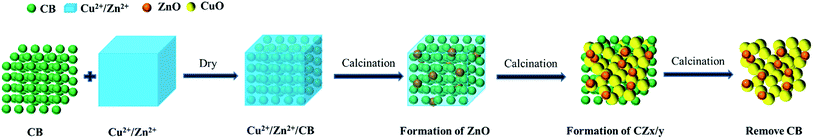 |
| | Fig. 6 Schematic illustration of the formation processes of CZx/y in the presence of CB. | |
Secondly, the (Cu(NH3)4)2+ and (Zn(NH3)4)2+ species are adsorbed on the surface of the added CB to form Cu2+/Zn2+/CB, because CB possesses a large quantity of negatively charged surface carboxyl groups, which have the strong ability to adsorb cations via electrostatic attraction. Subsequently, a low-temperature calcination is carried out which leads to the formation of ZnO nuclei first as verified by TG analysis. However, due to the confined effect of CB and influence of (Cu(NH3)4)2+, these formed particles seem not to grow fast enough. With the rise of calcination temperature, a large number of the CuO nuclei are generated by decomposition of (Cu(NH3)4)2+ and their growth occurs on the surface of the pre-formed ZnO NPs. Finally, after the high-temperature calcination, the CB template is removed to produce honeycomb-like CZx/y NPs. CB in this process not only serves as an aggregation-inhibiting agent but also as a structure-directing agent for the formation of honeycomb-like structure. As observed above, in the absence of CB, the obtained crystals are composed of large and dense particles without voids among them. The relevant chemical reactions involved in this process can be presented as follows:
| | |
Cu + 2CH3COONH4 + 2NH4OH → (Cu(NH3)4)2+ + 2CH3COO− + 3H2O
| (2) |
| | |
CuO + CH3COONH4 + 3NH4OH → (Cu(NH3)4)2+ + CH3COO− + 4H2O
| (3) |
| | |
Zn + 2CH3COONH4 + 2NH4OH → (Zn(NH3)4)2+ + 2CH3COO− + 3H2O
| (4) |
| | |
(Zn(NH3)4)2+ + 2CH3COO− → ZnO + 4NH3↑ + 2CH4↑
| (5) |
| | |
(Cu(NH3)4)2+ + 2CH3COO− → CuO + 4NH3↑ + 2CH4↑
| (6) |
Fig. 7 displays the temperature-programmed reduction (H2-TPR) profiles of the as-prepared CZ100/0, CZ95/5, CZ80/20, CZ50/50 and CZ0/100 samples with 67 wt% CB. The peak temperatures (TM) are compiled in Table 1. For CZ100/0, there is only one broad reduction peak in the temperature range of 265–440 °C, which is assigned to the reduction step of CuO to Cu, consistent with our previous report.41 As compared to that of CZ100/0, the reduction peak of CZx/y samples shifts to lower temperature and its integral area becomes smaller with increasing ZnO content, and the lowest reduction temperature is observed at 259.4 °C for CZ50/50. In addition, the reduction temperature range of CZx/y samples becomes narrower significantly. These results confirm that the reducibility of CuO in CZx/y catalysts can be improved by adding Zn species.
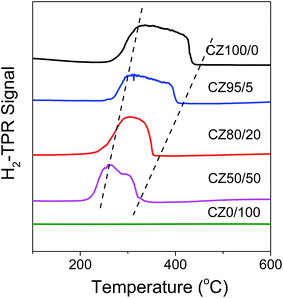 |
| | Fig. 7 H2-TPR curves of all the samples. | |
Fig. 8 displays the Cu 2p, Zn 2p, and O 1s XPS spectra of CZ100/0, CZ80/20 and CZ0/100 prepared with 67 wt% CB. The wide range XPS spectrum of the CZ80/20 sample verifies the presence of Cu, O, and Zn elements (Fig. 8a). The high-resolution XPS spectra of Cu 2p with binding energy calibrated with C 1s = 284.8 eV is shown in Fig. 8b. For CZ100/0, the major peaks at 962.9 eV and 954.1 eV are assigned to Cu 2p1/2, while the one at 934.2 eV is attributed to Cu 2p3/2, and the peak at ca. 943.8 eV corresponds to the satellite peak of CuO.42 Comparing with that of CuO, there is a slight shift of the major peak to lower binding energy for CZ80/20, indicating the electron density on CuO species is modified due to the interactive coupling between CuO and ZnO NPs. Fig. 8c shows the high-resolution Zn 2p XPS spectrum. It can be seen that for CZ0/100, the binding energies of Zn 2p3/2 and Zn 2p1/2 are respectively located at 1021.9 eV and 1045.1 eV, indicating that the Zn species exists in the form of Zn2+.43 But in the case of CZ80/20 sample, the observation of the higher binding energies compared with those of the CZ0/100 demonstrates the electron-deficient state of Zn. Therefore, the above observed peak shifts should be ascribed to charge transfer at the interface between ZnO and CuO, confirming the presence of strong synergistic interaction in CZ80/20 sample, which may improve the catalytic performance as compared with that of pristine ZnO NPs or CuO NPs alone.44 The XPS spectra of O 1s are presented in Fig. 8d, in which the binding energies of O 1s for CZ100/0, CZ80/20 and CZ0/100 are different, suggesting that the addition of Zn species alters the binding energy of O 1s obviously.
 |
| | Fig. 8 XPS survey spectra of CZ80/20 (a), Cu 2p spectrum of CZ100/0 and CZ80/20 samples (b), Zn 2p spectrum of CZ80/20 and CZ0/100 samples (c), and O 1s spectrum of CZ100/0, CZ80/20 and CZ0/100 samples (d). | |
Table 2 shows the catalytic results of all the samples for M2 synthesis via the Rochow reaction. As shown, the CZx/y samples prepared in the presence of CB is more active in comparison with that prepared in the absence of CB even with the same Cu and Zn weight ratio. For example, CZ100/0 obtained without CB exhibits a low M2 selectivity of 55.4% and Si conversion of 8.1%; while for CZ100/0 obtained with CB, the corresponding values become 67.2 and 14.8% respectively under identical reaction conditions. For the samples prepared with 67% CB, the catalytic performances are further improved with addition of more ZnO when the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio is below 20
Cu ratio is below 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80. A remarkably improved M2 selectivity of 88.7% is achieved based on a Si conversion of 23.5% in the case of CZ80/20 sample. However, the catalyst performance becomes poorer dramatically again with the further increase of Zn
80. A remarkably improved M2 selectivity of 88.7% is achieved based on a Si conversion of 23.5% in the case of CZ80/20 sample. However, the catalyst performance becomes poorer dramatically again with the further increase of Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio, and the M2 selectivity and Si conversion decrease to 39.5 and 2.2% respectively when the Zn
Cu ratio, and the M2 selectivity and Si conversion decrease to 39.5 and 2.2% respectively when the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio reaches 50
Cu ratio reaches 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. This is possibly because the excessive ZnO reduces the contact of the catalyst active component CuO with the Si powder. In addition, a shorter induction period is observed for CZ80/20 compared to other samples. For comparison, a commercial catalyst, e.g., Cu–Cu2O–CuO (SEM image shown in Fig. S5 in ESI†) is also employed. It can be seen that the catalytic property of CZ80/20 catalyst is close to that of the commercial Cu–Cu2O–CuO catalyst (with 82.3% M2 selectivity and 24.4% Si conversion), although the latter has a more complex composition than the former. It should be pointed out that the experimental errors for both conversion and selectivity are ±0.1% and all the catalytic results are obtained by three repeated experiments under the same conditions. As we know, a high M2 selectivity and yield is highly desired in organosilane industry. These results demonstrate that the amount of CB and Zn species have great effect on the catalytic performance, and the prepared CZ80/20 catalyst in the presence of 67% CB possesses the best catalytic performance among all the samples tested. This may be attributed to its smaller crystal size and honeycomb-like structure together with the synergistic effect between CuO and ZnO, which can promote the catalytic activity for the Rochow reaction. Our previous studies have also revealed the presence of this synergistic effect.44 In addition, byproducts such as M1, M3, M1H, M2H, LB, and HB over the various prepared catalysts are also detected.
50. This is possibly because the excessive ZnO reduces the contact of the catalyst active component CuO with the Si powder. In addition, a shorter induction period is observed for CZ80/20 compared to other samples. For comparison, a commercial catalyst, e.g., Cu–Cu2O–CuO (SEM image shown in Fig. S5 in ESI†) is also employed. It can be seen that the catalytic property of CZ80/20 catalyst is close to that of the commercial Cu–Cu2O–CuO catalyst (with 82.3% M2 selectivity and 24.4% Si conversion), although the latter has a more complex composition than the former. It should be pointed out that the experimental errors for both conversion and selectivity are ±0.1% and all the catalytic results are obtained by three repeated experiments under the same conditions. As we know, a high M2 selectivity and yield is highly desired in organosilane industry. These results demonstrate that the amount of CB and Zn species have great effect on the catalytic performance, and the prepared CZ80/20 catalyst in the presence of 67% CB possesses the best catalytic performance among all the samples tested. This may be attributed to its smaller crystal size and honeycomb-like structure together with the synergistic effect between CuO and ZnO, which can promote the catalytic activity for the Rochow reaction. Our previous studies have also revealed the presence of this synergistic effect.44 In addition, byproducts such as M1, M3, M1H, M2H, LB, and HB over the various prepared catalysts are also detected.
Table 2 Catalytic performances of all the catalysts for Rochow reactiona
| Samples |
Product composition (%) |
C–Si (%) |
| M1 |
M2 |
M3 |
M1H |
M2H |
LBR |
HBR |
Reaction conditions: cat., 0.5 g; catalyst: Si (mass ratio) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; CH3Cl flow, 25 mL min−1; temp., 325 °C; time, 24 h. 20; CH3Cl flow, 25 mL min−1; temp., 325 °C; time, 24 h. |
| CB 0 wt% |
CZ100/0 |
26.7 |
55.4 |
2.9 |
12.1 |
0.3 |
0.6 |
2.0 |
8.1 |
| CZ95/5 |
25.5 |
59.2 |
2.1 |
10.8 |
0.5 |
0.6 |
1.3 |
9.9 |
| CZ80/20 |
22.5 |
64.7 |
1.8 |
9.3 |
0.5 |
0.5 |
0.7 |
10.5 |
| CZ50/50 |
45.7 |
28.4 |
0.1 |
22.8 |
0.7 |
0.7 |
1.6 |
0.3 |
| CZ0/100 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| CB 67 wt% |
CZ100/0 |
21.8 |
67.2 |
1.8 |
6.4 |
0.7 |
0.9 |
1.2 |
14.8 |
| CZ95/5 |
13.8 |
78.5 |
1.2 |
3.3 |
2.2 |
0.6 |
0.4 |
17.8 |
| CZ80/20 |
2.6 |
88.7 |
1.2 |
4.4 |
1.9 |
0.5 |
0.7 |
23.5 |
| CZ50/50 |
37.4 |
39.5 |
1.6 |
20.0 |
0.4 |
0.3 |
0.8 |
2.2 |
| CZ0/100 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Commercial Cu–Cu2O–CuO |
5.8 |
82.3 |
1.3 |
4.8 |
1.2 |
0.3 |
4.3 |
24.4 |
The effects of the reaction temperature on the performance of CZ80/20 catalyst are investigated. For comparison, other CZx/y samples are also tested, and the results are shown in Fig. 9. Obviously, the CZx/y samples prepared in the presence of CB exhibit much better catalytic performance than the ones prepared in the absence of CB. On the other hand, for all CZx/y samples prepared in the presence of CB, the M2 selectivity increases with increasing reaction temperature from 295 to 325 °C. Afterwards, it becomes lower with further increase of the temperature from 325 to 355 °C. Furthermore, the Si conversion is improved continuously with increase of the temperature, reaching the maximum value at 325 °C. For CZ100/0 prepared in the presence of CB, the maximum Si conversion and M2 selectivity at 325 °C is 8.1% and 55.4%, respectively. After the Zn addition, the activity of CZx/y is significantly improved compared with CZ100/0 except for CZ50/50. Among them, CZ80/20 exhibits the best catalytic performance, achieving Si conversion of 23.5% and M2 selectivity of 88.7% at 325 °C. The above results indicate that the reaction temperature has an obvious influence on the catalytic performance of the CZx/y samples, and among them, CZ80/20 is still the most efficient catalyst for selective synthesis of M2 product. The temperature might effect on the activation energy, thereby leading to the change of reaction kinetics, as reported in the previous reports.44–46
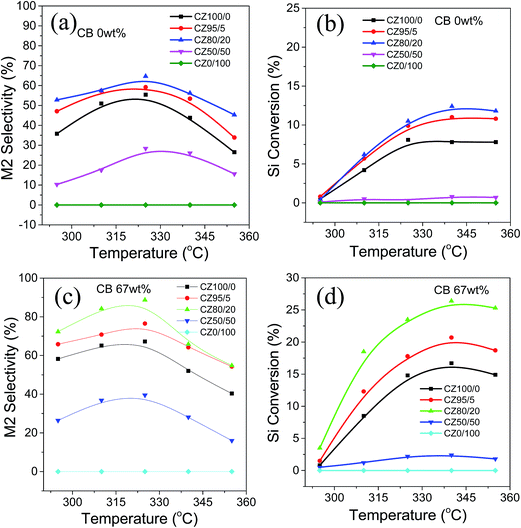 |
| | Fig. 9 M2 selectivity and Si conversion of CZx/y with different amount of CB at various reaction temperatures for the Rochow reaction. Reaction conditions: temp., 295–355 °C; others, see Table 2. | |
Fig. 10 displays the XRD patterns of the waste contact masses after the reaction, which contains the unreacted Si and the CZx/y catalysts. As indicated, all the waste contact masses are composed of Si, Cu and ZnO and there is no detection of any CuO (Fig. 10a and c). The metallic Cu may originate from the reaction of MeCl with the lattice oxygen of the CZx/y catalyst and O atoms in organic or inorganic compounds as the reaction proceeds.47 An enlarged view of the XRD patterns in the range of 42–50° (Fig. 10b and d) shows the presence of Cu3Si species, suggesting the formation of alloyed CuxSi active components through reaction of Cu and Si via diffusion during the reaction. In the Rochow reaction, Cu3Si is a metal alloy of η phase which has high activity towards MeCl,48 and is normally generated between the Cu catalyst and the Si interface at elevated temperatures. With the formation of Cu3Si and its interaction with MeCl molecules, the latter are polarized, which is conductive to the generation of MCSs, especially M2. The amount of Cu3Si can substantially affect the Si conversion and M2 selectivity.48 Although the intensity of Cu peaks for all the waste contact masses are similar, a higher intensity of CuxSi is observed in the case of CZ80/20 prepared in the presence of CB than that of the other samples, suggesting the former more active in generating CuxSi than the other samples. The SEM image of the contact masses for CZ80/20 sample after the reaction shows that the Si surface became coarse or porous (Fig. 11a), suggesting the occurrence of etching process during the reaction, consistent with the so-called anisotropic etching reaction mechanism.35,41,49 These observations indicate that the CZ80/20 catalyst is very active for the Rochow reaction. The element mapping images reveals the distribution of the elements Si (Fig. 11b), Cu (Fig. 11c), Zn (Fig. 11d), Cl (Fig. 11e) and C (Fig. 11f) on the surface of the waste contact mass. As indicated, the Si mapping image clearly shows the presence of the reacted (dark red) and unreacted (bright red) zones of Si particles, while Cu (bright green), Zn (bright yellow), Cl (bright violet) and C (bright red) distribute uniformly on the reacted or etched Si surface, indicating the occurrence of catalytic reaction between Si particles and Cu-based catalysts. The EDS spectrum (Fig. 11g) also demonstrates the presence of C, O, Si, Cl, and Zn element, of which C may come from decomposition of MeCl.50 On the basis of these experimental studies and characterizations, we thus propose the catalytic mechanism for Rochow reaction on the CZx/y hybrid catalysts, which is shown in Fig. 11h. First, the CZx/y samples are reduced to generate metallic Cu in the induction period, which is due to the reaction of CH3Cl with the lattice oxygen in CuO (XRD result in Fig. 10a and c). Then the metallic Cu species diffuses into the Si interior forming an interface composed of Si, Cu, and CuxSi phases. Among these species, CuxSi formed is an indicator of the activity of a Cu-based catalyst and is normally suggested as the key catalytic active intermediate, on which, MCSs especially M2 are formed because M2 is thermodynamically more stable than the other monomers. We thus conclude that the higher Si conversion and M2 selectivity of CZ80/20 sample compared to the other catalysts is due to the following factors: (1) the faster gas transportation of MeCl within this sample because of honeycomb-like structure; (2) the proper composition of CuO and ZnO creates a stronger synergistic effect; (3) generation of larger interfaces in this sample and the Si surface because of its smaller crystal size. All these factors will contribute to the formation of more CuxSi species as shown in Fig. 10c, thus promoting the catalytic activity for M2 production.
 |
| | Fig. 10 XRD patterns of waste contact masses (a and c) and enlarged view in the 2θ range of 40–50° (b and d). | |
 |
| | Fig. 11 SEM images of Si particle after reaction (a), elemental mapping images of Si (b), Cu (c), Zn (d), Cl (e), C (f), SEM-EDX spectra (g) and schematic illustration of the Rochow reaction process (h). | |
4. Conclusions
In summary, a series of CZx/y honeycomb-like nano-hybrids with CuO NPs adhered with ZnO NPs have been successfully prepared through adsorption of Cu2+/Zn2+ ions on CB followed with calcination in air. In this process, the Cu2+/Zn2+ ions are obtained from the waste contact masses via ammonia leaching and the CB not only acts as the agglomeration inhibitor but also as the hard template. When applied to the Rochow reaction, the as-synthesized CZ80/20 NPs exhibit a M2 selectivity of 88.7% and Si conversion of 23.5%, much higher than those of CuO/ZnO NPs prepared without CB, the sole CuO or ZnO NPs and the CuO/ZnO NPs with different composition. Extensive characterizations indicate this improved catalytic performance is related to the honeycomb-like structure, the smaller Cu crystal size as well as synergistic electronic effect between Cu and ZnO in the CZ80/20 NPs catalyst. This work not only develops a low cost and scalable method for preparing hierarchically structured CuO/ZnO material, but also provides a route for effectively utilizing the copper component in the waste contact masses.
Acknowledgements
The authors gratefully acknowledge the financial supports from the National Natural Science Foundation of China (No. 21506224, and 51272252). Z. Zhong is mainly working in Institute of Chemical and Engineering Sciences (ICES) in Singapore, and also holds a part-time position in Nanyang Environment & Water Research Institute (NEWRI) in Nanyang Technological University. He would like to thank ICES for the kind support of the collaboration.
References
- S. Allahyari, M. Haghighi, A. Ebadi and H. Qavam Saeedi, J. Power Sources, 2014, 272, 929–939 CrossRef CAS.
- A. Kargar, Y. Jing, S. J. Kim, C. T. Riley, X. Pan and D. Wang, ACS Nano, 2013, 7, 11112–11120 CrossRef CAS PubMed.
- Q. Tang, L. Lin, X. Zhao, K. Huang and J. Wu, Langmuir, 2012, 28, 3972–3978 CrossRef CAS PubMed.
- F. Schuster, B. Laumer, R. R. Zamani, C. Magén, J. R. Morante, J. Arbiol and M. Stutzmann, ACS Nano, 2014, 8, 4376–4384 CrossRef CAS PubMed.
- M. M. Rahman and A. M. Asiri, Sens. Actuators, B, 2015, 214, 82–91 CrossRef CAS.
- I. M. Ibrahim, S. I. Sharhan and F. T. Ibrahim, Mater. Lett., 2015, 157, 57–62 CrossRef CAS.
- W. N. Wang, F. Wu, Y. Myung, D. M. Niedzwiedzki, H. S. Im, J. Park, P. Banerjee and P. Biswas, ACS Appl. Mater. Interfaces, 2015, 7, 5685–5692 Search PubMed.
- Z. Guo, X. Chen, J. Li, J. H. Liu and X. J. Huang, Langmuir, 2011, 27, 6193–6200 CrossRef CAS PubMed.
- N. Datta, N. S. Ramgir, S. Kumar, P. Veerender, M. Kaur, S. Kailasaganapathi, A. K. Debnath, D. K. Aswal and S. K. Gupta, Sens. Actuators, B, 2014, 202, 1270–1280 CrossRef CAS.
- A. Katoch, S.-W. Choi, J.-H. Kim, J. H. Lee, J.-S. Lee and S. S. Kim, Sens. Actuators, B, 2015, 214, 111–116 CrossRef CAS.
- L. Wang, Y. Kang, Y. Wang, B. Zhu, S. Zhang, W. Huang and S. Wang, Mater. Sci. Eng., C, 2012, 32, 2079–2085 CrossRef CAS.
- Y. Zhu, C. H. Sow, T. Yu, Q. Zhao, P. Li, Z. Shen, D. Yu and J. T. L. Thong, Adv. Funct. Mater., 2006, 16, 2415–2422 CrossRef CAS.
- M. Eshed, J. Lellouche, A. Gedanken and E. Banin, Adv. Funct. Mater., 2014, 24, 1382–1390 CrossRef CAS.
- J.-H. Kim, A. Katoch and S. S. Kim, Sens. Actuators, B, 2016, 222, 249–256 CrossRef CAS.
- M. Yin, F. Wang, H. Fan, L. Xu and S. Liu, J. Alloys Compd., 2016, 672, 374–379 CrossRef CAS.
- K. Mageshwari, D. Nataraj, T. Pal, R. Sathyamoorthy and J. Park, J. Alloys Compd., 2015, 625, 362–370 CrossRef CAS.
- S. Jung and K. Yong, Chem. Commun., 2011, 47, 2643–2645 RSC.
- W. C. Huang, L. M. Lyu, Y. C. Yang and M. H. Huang, J. Am. Chem. Soc., 2012, 134, 1261–1267 CrossRef CAS PubMed.
- A. Zainelabdin, G. Amin, S. Zaman, O. Nur, J. Lu, L. Hultman and M. Willander, J. Mater. Chem., 2012, 22, 11583 RSC.
- S. Pukird, W. Song, S. Noothongkaew, S. K. Kim, B. K. Min, S. J. Kim, K. W. Kim, S. Myung and K.-S. An, Appl. Surf. Sci., 2015, 351, 546–549 CrossRef CAS.
- B. Behera and S. Chandra, J. Mater. Sci. Technol., 2015, 31, 1069–1078 Search PubMed.
- T. Terasako, N. A. Hambali, N. A. Jayah, T. Wakisaka, A. M. Hashim and M. Yagi, Thin Solid Films, 2015, 596, 201–208 CrossRef CAS.
- T. Terasako, T. Murakami, A. Hyodou and S. Shirakata, Sol. Energy Mater. Sol. Cells, 2015, 132, 74–79 CrossRef CAS.
- R. Shi, P. Yang, S. Zhang and X. Dong, Ceram. Int., 2014, 40, 3637–3646 CrossRef CAS.
- J. Wang, W.-D. Zhang, W.-X. Ouyang and Y.-X. Yu, Mater. Lett., 2015, 154, 44–46 CrossRef.
- X. Chen, Y. Huang, X. Zhang, C. Li, J. Chen and K. Wang, Mater. Lett., 2015, 152, 181–184 CrossRef CAS.
- D. H. Sun, B. E. Bent, A. P. Wright and B. M. Naasz, J. Mol. Catal. A: Chem., 1998, 131, 169–183 CrossRef CAS.
- T. J. Wessel and D. G. Rethwisch, J. Catal., 1996, 161, 861–866 CrossRef CAS.
- D. Seyferth, Organometallics, 2001, 20, 4978–4992 CrossRef CAS.
- L. N. Lewis and W. J. Ward, Ind. Eng. Chem. Res., 2002, 41, 397–402 CrossRef CAS.
- J. Yu, H. Zhan, Y. Wang, Z. Zhang, H. Chen, H. Li, Z. Zhong and F. Su, J. Power Sources, 2013, 228, 112–119 CrossRef CAS.
- Z. Z. Zhang, Y. H. Wang, W. F. Ren, Q. Q. Tan, Y. F. Chen, H. Li, Z. Y. Zhong and F. B. Su, Angew. Chem., Int. Ed., 2014, 53, 5165–5169 CAS.
- L. Shi, W. Wang, A. Wang, K. Yuan and Y. Yang, J. Mater. Chem. A, 2014, 2, 20213–20220 RSC.
- J. Y. Wu, W. S. Chou, W. S. Chen, F. C. Chang, Y. H. Shen, J. E. Chang and M. S. Tsai, Desalin. Water Treat., 2012, 47, 120–129 CrossRef CAS.
- Z. Z. Zhang, H. W. Che, Y. L. Wang, X. L. She, J. Sun, P. Gunawan, Z. Y. Zhong and F. B. Su, ACS Appl. Mater. Interfaces, 2012, 4, 1295–1302 Search PubMed.
- Y. Zhou, X. Sun, K. Zhong, D. G. Evans, Y. Lin and X. Duan, Ind. Eng. Chem. Res., 2012, 51, 4215–4221 CrossRef CAS.
- C. Jeong, M. J. Hyun and Y.-W. Suh, Catal. Commun., 2015, 70, 34–39 CrossRef CAS.
- S. Kudo, T. Maki, K. Miura and K. Mae, Carbon, 2010, 48, 1186–1195 CrossRef CAS.
- S. Muthukumaran and R. Gopalakrishnan, Opt. Mater., 2012, 34, 1946–1953 CrossRef CAS.
- V. Gargiulo, M. Alfè, P. Ammendola, F. Raganati and R. Chirone, Appl. Surf. Sci., 2016, 360, 329–337 CrossRef CAS.
- S. M. Liu, Y. L. Wang, Y. X. Zhu, G. N. Wang, Z. L. Zhang, H. W. Che, L. H. Jia and F. B. Su, RSC Adv., 2014, 4, 7826–7833 RSC.
- D. Wang, L. Zhang, L. Chen, H. Wu and P. Wu, J. Mater. Chem. A, 2015, 3, 3511–3521 RSC.
- Y. X. Zhu, Y. L. Wang, L. Y. Song, X. Chen, W. Y. Liu, J. Sun, X. L. She, Z. Y. Zhong and F. B. Su, RSC Adv., 2013, 3, 9794–9806 RSC.
- A. D. Gordon, B. J. Hinch and D. R. Strongin, J. Catal., 2009, 266, 291–298 CrossRef CAS.
- J. G. Rocha Poço, H. Furlan and R. Giudici, J. Phys. Chem. B, 2002, 106, 4873–4877 CrossRef.
- M. G. R. T. De Cooker, J. W. De Jong and P. J. Van Den Berg, J. Organomet. Chem., 1975, 86, 175–183 CrossRef CAS.
- L. N. Lewis, W. V. Ligon and J. C. Carnahan, Silicon Chem., 2002, 1, 23–33 CrossRef CAS.
- L. Stolt, F. M. D'Heurle and J. M. E. Harper, Thin Solid Films, 1991, 200, 147–156 CrossRef CAS.
- G. Liu, K. L. Young, X. Liao, M. L. Personick and C. A. Mirkin, J. Am. Chem. Soc., 2013, 135, 12196–12199 CrossRef CAS PubMed.
- Z. L. Zhang, H. W. Che, J. J. Gao, Y. L. Wang, X. L. She, J. Sun, P. Gunawan, Z. Y. Zhong and F. B. Su, Catal. Sci. Technol., 2012, 2, 1207–1212 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11132g |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were mixed homogeneously to form a contact mass, which was loaded in the glass reactor. The reactor system was purged with purified N2 for 0.5 h followed by heating to 295–355 °C within 1 h under a N2 flow rate of 25 mL min−1. Subsequently, N2 was turned off, and CH3Cl gas with a flow rate of 25 mL min−1 was introduced into the reactor to react with Si followed by heating to 325 °C. After a given period of 24 h, the reaction was stopped. The gas product was cooled to liquid phase with the circulator bath controlled at −5 °C by a programmable thermal circulator (GDH series, Ningbo xinzhi biological technology Co., LTD). The products in the liquid solution were quantitatively analyzed on an Agilent Technologies (GC-7890A) gas chromatograph equipped with KB-201 column (60 m) and TCD detector. Gas chromatography-mass spectrometer system (GC-MS) was used for identification of the products, which were mainly comprised of methyltrichlorosilane (CH3SiCl3, M1), dimethyldichlorosilane ((CH3)2SiCl2, M2), trimethylchlorosilane ((CH3)3SiCl, M3), methyldichlorosilane (CH3SiHCl2, M1H), dimethylchlorosilane ((CH3)2SiHCl, M2H), low boiler (LB) and high boiler (HB). The selectivity of the products was calculated by the peak area ratio (in percentage). The spent contact masses (residual solid after reaction) containing unreacted Si powder and CZx/y compounds were weighed to obtain the ratio of weight difference of contact mass (before and after reaction) and weight of Si (before reaction) for the purpose of calculating the Si conversion (formula (1)).
1 were mixed homogeneously to form a contact mass, which was loaded in the glass reactor. The reactor system was purged with purified N2 for 0.5 h followed by heating to 295–355 °C within 1 h under a N2 flow rate of 25 mL min−1. Subsequently, N2 was turned off, and CH3Cl gas with a flow rate of 25 mL min−1 was introduced into the reactor to react with Si followed by heating to 325 °C. After a given period of 24 h, the reaction was stopped. The gas product was cooled to liquid phase with the circulator bath controlled at −5 °C by a programmable thermal circulator (GDH series, Ningbo xinzhi biological technology Co., LTD). The products in the liquid solution were quantitatively analyzed on an Agilent Technologies (GC-7890A) gas chromatograph equipped with KB-201 column (60 m) and TCD detector. Gas chromatography-mass spectrometer system (GC-MS) was used for identification of the products, which were mainly comprised of methyltrichlorosilane (CH3SiCl3, M1), dimethyldichlorosilane ((CH3)2SiCl2, M2), trimethylchlorosilane ((CH3)3SiCl, M3), methyldichlorosilane (CH3SiHCl2, M1H), dimethylchlorosilane ((CH3)2SiHCl, M2H), low boiler (LB) and high boiler (HB). The selectivity of the products was calculated by the peak area ratio (in percentage). The spent contact masses (residual solid after reaction) containing unreacted Si powder and CZx/y compounds were weighed to obtain the ratio of weight difference of contact mass (before and after reaction) and weight of Si (before reaction) for the purpose of calculating the Si conversion (formula (1)).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn weight ratios determined by ICP-OES verify that the compositions of the final products are close to the initial feeding ratio of the components, as shown in Table 1.
Zn weight ratios determined by ICP-OES verify that the compositions of the final products are close to the initial feeding ratio of the components, as shown in Table 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio is below 20
Cu ratio is below 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80. A remarkably improved M2 selectivity of 88.7% is achieved based on a Si conversion of 23.5% in the case of CZ80/20 sample. However, the catalyst performance becomes poorer dramatically again with the further increase of Zn
80. A remarkably improved M2 selectivity of 88.7% is achieved based on a Si conversion of 23.5% in the case of CZ80/20 sample. However, the catalyst performance becomes poorer dramatically again with the further increase of Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio, and the M2 selectivity and Si conversion decrease to 39.5 and 2.2% respectively when the Zn
Cu ratio, and the M2 selectivity and Si conversion decrease to 39.5 and 2.2% respectively when the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio reaches 50
Cu ratio reaches 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. This is possibly because the excessive ZnO reduces the contact of the catalyst active component CuO with the Si powder. In addition, a shorter induction period is observed for CZ80/20 compared to other samples. For comparison, a commercial catalyst, e.g., Cu–Cu2O–CuO (SEM image shown in Fig. S5 in ESI†) is also employed. It can be seen that the catalytic property of CZ80/20 catalyst is close to that of the commercial Cu–Cu2O–CuO catalyst (with 82.3% M2 selectivity and 24.4% Si conversion), although the latter has a more complex composition than the former. It should be pointed out that the experimental errors for both conversion and selectivity are ±0.1% and all the catalytic results are obtained by three repeated experiments under the same conditions. As we know, a high M2 selectivity and yield is highly desired in organosilane industry. These results demonstrate that the amount of CB and Zn species have great effect on the catalytic performance, and the prepared CZ80/20 catalyst in the presence of 67% CB possesses the best catalytic performance among all the samples tested. This may be attributed to its smaller crystal size and honeycomb-like structure together with the synergistic effect between CuO and ZnO, which can promote the catalytic activity for the Rochow reaction. Our previous studies have also revealed the presence of this synergistic effect.44 In addition, byproducts such as M1, M3, M1H, M2H, LB, and HB over the various prepared catalysts are also detected.
50. This is possibly because the excessive ZnO reduces the contact of the catalyst active component CuO with the Si powder. In addition, a shorter induction period is observed for CZ80/20 compared to other samples. For comparison, a commercial catalyst, e.g., Cu–Cu2O–CuO (SEM image shown in Fig. S5 in ESI†) is also employed. It can be seen that the catalytic property of CZ80/20 catalyst is close to that of the commercial Cu–Cu2O–CuO catalyst (with 82.3% M2 selectivity and 24.4% Si conversion), although the latter has a more complex composition than the former. It should be pointed out that the experimental errors for both conversion and selectivity are ±0.1% and all the catalytic results are obtained by three repeated experiments under the same conditions. As we know, a high M2 selectivity and yield is highly desired in organosilane industry. These results demonstrate that the amount of CB and Zn species have great effect on the catalytic performance, and the prepared CZ80/20 catalyst in the presence of 67% CB possesses the best catalytic performance among all the samples tested. This may be attributed to its smaller crystal size and honeycomb-like structure together with the synergistic effect between CuO and ZnO, which can promote the catalytic activity for the Rochow reaction. Our previous studies have also revealed the presence of this synergistic effect.44 In addition, byproducts such as M1, M3, M1H, M2H, LB, and HB over the various prepared catalysts are also detected.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; CH3Cl flow, 25 mL min−1; temp., 325 °C; time, 24 h.
20; CH3Cl flow, 25 mL min−1; temp., 325 °C; time, 24 h.










