DOI:
10.1039/C6RA11112B
(Paper)
RSC Adv., 2016,
6, 73959-73973
Core–shell zeolite@Alg–Ca particles for removal of strontium from aqueous solutions
Received
29th April 2016
, Accepted 4th July 2016
First published on 5th July 2016
Abstract
A core–shell zeolite@Alg–Ca adsorbent was synthesized by a simple method of coaxial electrospinning and applied for the removal of Sr(II) ions from aqueous solution. The effects of various synthesis parameters, CaCl2 concentration and voltage on the ability of the adsorbent to remove Sr(II) ions were studied. These parameters were found to have a significant influence on the adsorption capacity of the adsorbent for Sr(II) ions. The influence of several parameters such as pH, contact time, adsorbent dosage, initial strontium concentration, co-existing cation and temperature on the adsorption of Sr(II) ions were also investigated. The experimental data were analyzed using adsorption isotherm and adsorption kinetics. The adsorption isotherm was well described by the linear Freundlich isotherm model and the adsorption kinetics were well fitted by a linear pseudo-second-order model. The maximum adsorption capacity of the adsorbent for Sr(II) ions was 83.31 mg g−1 at pH 4 and 328.15 K. The presence of Na(I) ions, Mg(II) ions, UO2(II) ions, K(I) ions, and particularly Ca(II) ions, in the concentration range of 100–1000 mg L−1 had a large negative impact on the removal of Sr(II) ions. The strontium adsorption process was feasible and spontaneous. The core–shell zeolite@Alg–Ca particles were more stable than zeolite in both acid and alkali media. The adsorption of Sr(II) ions on the adsorbent was realized via ion exchange and the adsorbent showed good reusability.
1. Introduction
The rapid development of nuclear power plants (NPPs) has led to an increase in attention to the treatment of low-level radioactive wastewater (LLRWs).1 Radioactive strontium is one of the most abundant contaminants in LLRWs on account of its long half-life, high solubility and transferability.2 Because the chemical properties of strontium are similar to calcium, Sr(II) ions can easily replace Ca(II) ions in human bodies and cause leukemia, anemia and other diseases.3 As a result, the removal of Sr(II) ions from waste solutions is very important.
Until now, the removal of Sr(II) ions from aqueous solutions has been achieved by precipitation,4 membrane filtration,5 biological processes6 and adsorption.7 Among the different treatment methods, adsorption is the most widely used method to treat Sr(II) ion-contaminated wastewater owing to its efficiency and simplicity in operation.
Alginate is a polysaccharide, and has the advantage of being an abundant resource with a low cost and biodegradability. Alginate is widely used as an adsorbent for the removal of various metal ions because of its many carboxylic groups, and the high affinity of carboxylic groups for metal ions.8 Moreover, alginate is a good immobilizer that shows excellent resistance to acids and alkalis, is non-toxic and has a variety of functional groups, such as hydroxy and carboxyl, that can interact with metal ions under mild conditions.9 However, alginate has some obvious disadvantages such as high degrees of swelling and water-solubility and poor removal capacity for Sr(II) ions. Therefore we prepared an adsorbent with Ca(II) ions as a crosslinking agent to form a stable and insoluble gel in water, this improves the stability of the adsorbent as well as enhancing the adsorption capacity between the Ca(II) ions and Sr(II) ions through cation exchange.10 This property of alginate is considered an advantage for a potential adsorbent used for the removal of metal ions.
Zeolite is crystalline, where tetrahedral AlO4 and SiO4 are linked together by sharing an oxygen atom. The presence of aluminium results in a negatively charged framework, which is neutralized by cations, mostly sodium ions. Cations such as strontium ions can easily be adsorbed onto zeolite by replacing the sodium ions associated with the crystals, showing that zeolite has a high ion exchange property for strontium ions.11,12 Hence, zeolite is a promising material for the removal of strontium.13 However, its small particle size and weak stability in acidic or alkali media make the recovery of the adsorbent after the adsorption of strontium ions difficult and restricts its practical applications.14
To overcome these disadvantages, core–shell zeolite@Alg–Ca particles are fabricated via coaxial electrospinning in this study. The particles contain an inner core of zeolite and an outer shell of alginate which increases the stability of the zeolite in acidic or alkali media and also provides active sites in alginate with carboxylic groups for the further adsorption of Sr(II) ions. Moreover, the core–shell zeolite@Alg–Ca particles can easily to be separated from solution after adsorption, which results in a reduced cost for the adsorption process and avoids secondary pollution.
The aims of this study were to (i) prepare core–shell zeolite@Alg–Ca particles by a safe and simple method. (ii) Investigate the effects of various synthesis conditions, such as CaCl2 concentration, voltage and adsorption factors, i.e. contact time, pH, initial strontium concentration, co-existing cations and temperature on the adsorption capacity of core–shell zeolite@Alg–Ca for Sr(II) ions by batch experiments. (iii) Study the adsorption isotherm, adsorption kinetics and the reusability of the adsorbent. (iv) Explore the mechanism of the strontium adsorption process.
2. Material and methods
2.1 Materials
Strontium chloride (SrCl2·6H2O), sodium alginate (NaAlg), anhydrous calcium chloride (CaCl2), cesium chloride (CsCl), magnesium chloride hexahydrate (MgCl2), sodium chloride (NaCl), potassium chloride (KCl) and dichloromethane (DCM) used in the experiments were of analytical grade, from Kelong Co., Ltd., and used without further purification. Uranium nitrate (UO2(NO3)2·6H2O) was obtained from HuBei Chushengwei Chemistry Co., Ltd. The zeolite (Na2O·Al2O3·xSiO2·yH2O) was purchased from Sinopharm Chemical Reagent Co., Ltd. The strontium stock solution was prepared by dissolving SrCl2·6H2O in distilled water. The solution pH was adjusted by adding 0.1 M HCl or 0.1 M NaOH in deionized water.
2.2 Synthesis of core–shell zeolite@Alg–Ca particles
The core–shell zeolite@Alg–Ca particles were obtained by coaxial electrospinning (DT-1003, Dalian, China), using a concentric nozzle (outer 16 # and inner 8 #) as the shell and core sprayer, respectively, as shown in the schematic in Fig. 1. The outer syringe contained sodium alginate aqueous solution at a concentration of 2 wt%. The inner syringe contained the core solution. The core solution was prepared by mixing 0.75 g of sodium alginate, 4.5 g of zeolite, 5 mL of dichloromethane with 50 mL of distilled water. Both the core and shell solutions were injected simultaneously through the concentric nozzle and dropped into calcium chloride (CaCl2) solution using a coaxial electrospinning machine at an applied voltage of 1 kV. The flow rate of the core solution and shell solution were maintained using a syringe pump at a rate of 0.02 mm s−1 and 0.025 mm s−1, respectively. The resulting particles were stirred for 24 h in the calcium chloride solution to allow crosslinking of the sodium alginate with the calcium ions through the exchange of the sodium-alginate sodium ions with calcium ions. Then, the solution was then filtered, washed with deionized water and dried at 40 °C in an air oven.
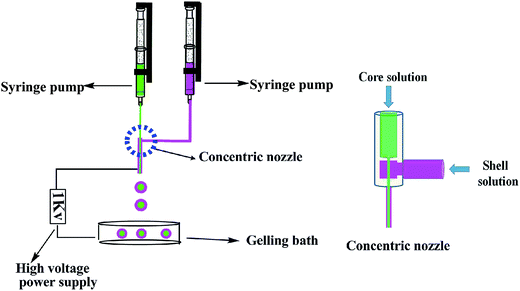 |
| | Fig. 1 Diagram of the coaxial electrospinning set-up and the concentric nozzle. | |
2.3 Batch adsorption experiments
Strontium removal performance was assessed through a batch equilibrium process. Batch adsorption experiments were carried out at various pH values, adsorbent doses, contact times, initial strontium concentrations and temperature. The experiments were conducted using a thermostated shaker rotating at a speed of 140 rpm. Typically, about 0.05 g of dry adsorbent was added into 50 mL of 20 mg L−1 strontium solution at a desired pH value. The pH values ranged from 2 to 11 and were adjusted by the addition of 0.1 M HCl or 0.1 M NaOH solutions. For the isotherm experiments, the initial strontium concentration varied from 20 mg L−1, 50 mg L−1, 80 mg L−1, 110 mg L−1 to 140 mg L−1, and a temperature range between 288.15 K to 328.15 K at an optimum pH value. The kinetic studies were conducted at a strontium concentration of 20 mg L−1 with 0.05 g of adsorbent in a 50 mL solution for a predetermined contact time. In the experiment assessing the effect of co-existing cations, the Sr(II) concentration was fixed at 20 mg L−1 and the concentrations of the co-existing cations Na(I) ions, Mg(II) ions, Cs(I) ions, UO2(II) ions, K(I) ions and Ca(II) ions ranged from 0 to 1000 mg L−1. Sr(II) adsorption was conducted in double component systems Sr/Na, Sr/Mg, Sr/Cs, Sr/UO2, Sr/K and Sr/Ca to determine the influence of different cations. The residual strontium concentration in solution was measured by means of an atomic adsorption spectrometer. All experiments were replicated three times and the average values were used for all calculations. The equilibrium adsorption capacity of the adsorbent was calculated according to the following equation.| |
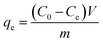 | (1) |
where qe is the adsorption capacity of the adsorbent (mg g−1), C0 and Ce are the initial and equilibrium concentrations (mg L−1), m (g) is the dry weight of the adsorbent and V (L) is the volume of strontium solution.
2.4 Regeneration and reusability of the adsorbent
Regeneration of the strontium saturated core–shell zeolite@Alg–Ca was conducted by the solvent desorption technique. Sr(II) desorption of the adsorbent was assessed using an HCl solution at pH 4 to regenerate the adsorbent. After reaching adsorption equilibrium, the adsorbent was separated, then added into an HCl solution at pH 4, and rotated at 140 rpm, 25 °C for 24 h. Finally, the adsorbent was washed with deionized water and the regenerated adsorbent (core–shell zeolite@Alg–Ca) was used again in the adsorption experiments. This process was repeated for up to three cycles. The regeneration efficiency (% RE) of adsorbent was calculated as follows:15| |
 | (2) |
where q0 and qr (mg g−1) are the adsorption capacities of the core–shell zeolite@Alg–Ca before and after regeneration.
2.5 Characterization of adsorbent
The physical/chemical characteristics of the core–shell zeolite@Alg–Ca adsorbent used in this study were evaluated using different technologies. The surface morphology of the core–shell zeolite@Alg–Ca adsorbent was examined by scanning electron microscopy (SEM, Ultra 55, Zesis Corporation), and then energy dispersive spectrometry (EDX) was employed to understand the elemental composition and distribution of the adsorbent before and after adsorption. X-ray photoelectron spectroscopy (XPS, Thermo Scientific Escalab 250, Thermo Fisher Corporation) was used to detect the binding energies of the adsorbent before and after adsorption.
3. Results and discussion
3.1 The effect of CaCl2 concentration on adsorption
Because the core–shell zeolite@Alg–Ca particles were synthesized by cross-linking sodium alginate with the CaCl2 solution, the concentration of the CaCl2 solution affects the structure, size and strength of the particles, and further affect the removal of strontium. In order to study the effect of the CaCl2 solution concentration on strontium adsorption, the concentration of CaCl2 was varied from 1 to 5 wt%. The results are shown in Fig. 2. It was observed that the 1 wt% CaCl2 solution was optimum, probably because the degree of crosslinking on the shell of the particles increased with increasing of Ca(II) ion concentration, resulting in the structure of the shell becoming more compact This hinders the strontium ions from entering and contacting the zeolite in the adsorbent core, resulting in a decrease in the Sr(II) ion adsorption capacity. Thus, the 1 wt% of CaCl2 concentration was chosen for core–shell zeolite@Alg–Ca particle preparation and was used for further studies.
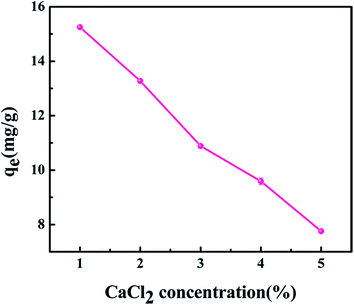 |
| | Fig. 2 The effect of CaCl2 concentration on strontium adsorption by core–shell zeolite@Alg–Ca particles (initial strontium concentration: 20 mg L−1, pH: 4, contact time: 24 h, temperature: 298.15 K, adsorbent dose: 0.05 g). | |
3.2 The effect of voltage and particle size on adsorption
Core–shell zeolite@Alg–Ca particles with different sizes were prepared at different voltages in the range of 1–9 kV and the effect of the particle size on strontium adsorption was investigated at a strontium concentration of 20 mg L−1. The results are shown in Fig. 3. It was found that the diameter of the particles decreased with increasing voltage (Fig. 3a), while the particle size of the adsorbent had little influence on the adsorption capacity (Fig. 3b). Considering resource utilization, a voltage of 1 kV was chosen for the preparation of core–shell zeolite@Alg–Ca particles.
 |
| | Fig. 3 The effect of (a) voltage on particle size and (b) particle size on strontium adsorption by core–shell zeolite@Alg–Ca particles (initial strontium concentration: 20 mg L−1, pH: 4, contact time: 24 h, temperature: 298.15 K, adsorbent dose: 0.05 g). | |
The results revealed that the optimal synthesis parameters for core–shell zeolite@Alg–Ca were 1 wt% CaCl2 concentration and a voltage of 1 kV.
3.3 Analysis of the resistance of the adsorbent to acid and alkali
The results of previous preliminary experiments showed that a solution pH of 2 to 11 was the critical range for the adsorbent resistance to acid and alkali conditions, and the best adsorption pH for strontium by the adsorbent was between 4 to 11. Therefore, pH of 2, 3, 4, 10 and 11 were chosen to evaluate the acid and alkali resistance of the adsorbent. Two types of adsorbents, for example, the zeolite and core–shell zeolite@Alg–Ca particles were soaked in solutions of different pHs for 7 d and the acid and alkali resistance results of the adsorbent are shown in Fig. 4. We can see from Fig. 4 that the zeolite adsorbent dissolved and the degree of dissolution increased with the increasing acidity or alkali of the solution (Fig. 4a1–a5). However, there was no significant change in the core–shell zeolite@Alg–Ca particles (Fig. 4b1–b5). This leads to the conclusion that the core–shell zeolite@Alg–Ca adsorbent shows better acid and alkali resistance, which may be attributed to the core–shell structure preventing the zeolite from dissolving in acid and alkali aqueous solutions.
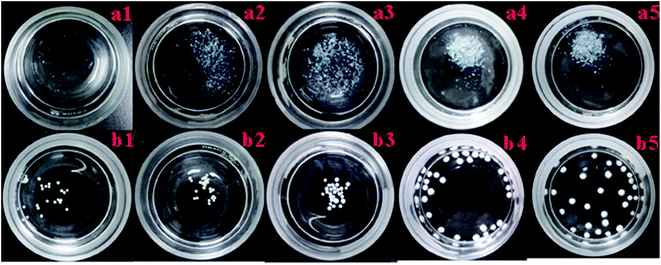 |
| | Fig. 4 Digital images of zeolite and core–shell zeolite@Alg–Ca at different pH values (a1, b1: pH 2; a2, b2: pH 3; a3, b3: pH 4, a4, b4: pH 10; a5, b5: pH 11 respectively). | |
3.4 Effect of pH on adsorption
It is known that pH is an important factor in the adsorption of metal cations.16 Based on the fact that Sr(II) ions will be hydrolyzed to SrOH+ at a pH higher than 11,17 the uptake of strontium by core–shell zeolite@Alg–Ca particles was investigated in the pH range of 2–11 and the results are presented in Fig. 5. The amount of strontium adsorbed on the core–shell zeolite@Alg–Ca particles, constant at pH 4 to 11, was equal to 20.60 mg g−1, while the strontium removal was observed to decrease in acidic medium, which is attributed to the excess of H(I) ions competing with the Sr(II) ions for adsorption sites.13
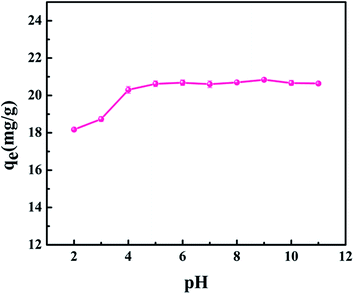 |
| | Fig. 5 Effect of solution pH on strontium adsorption by core–shell zeolite@Alg–Ca particles (initial strontium concentration: 20 mg L−1, contact time: 24 h, temperature: 298.15 K, adsorbent dose: 0.05 g). | |
3.5 Effect of adsorbent dose on adsorption
The influence of adsorbent dose on the removal of Sr(II) ions is shown in Fig. 6. It shows that the adsorption capacity of core–shell zeolite@Alg–Ca particles for Sr(II) ions decreased when the adsorbent dose increased from 0.01 to 0.11 g. The reduction in strontium adsorption may be due to the unsaturation of the adsorption active sites involved in the adsorption process.18,19 However, the removal rate of Sr(II) ions increased from 49.47% to 96.48% when the adsorbent dosage increased. This may be due to the fact that an increase in the adsorbent dose will provide more functional groups and active sites for the adsorption of Sr(II) ions, thus the removal rate of Sr(II) ions increases.20
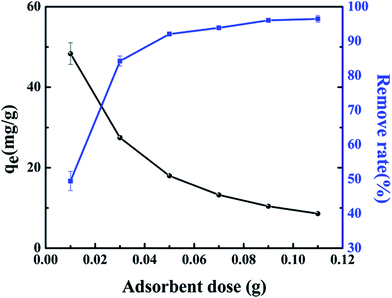 |
| | Fig. 6 Effect of adsorbent dose on strontium adsorption by core–shell zeolite@Alg–Ca adsorbent (initial strontium concentration: 20 mg L−1, pH: 4, contact time: 24 h, temperature: 298.15 K). | |
3.6 Effect of initial strontium concentration on adsorption
The relationship between the adsorption capacity and initial concentration of strontium is shown in Fig. 7. It was observed that the adsorption capacity of core–shell zeolite@Alg–Ca particles for Sr(II) ions increased as the initial strontium concentration increased, ranging from 20 mg L−1 to 120 mg L−1, and reaching a maximum of 88.31 mg g−1 at an initial concentration of 140 mg L−1 and 328.15 K, but the adsorption capacity increased slowly at higher concentrations. This may be attributed to the fact that more active sites of the adsorbent were involved in the adsorption process at lower concentrations and less coordination sites were available when the adsorption tended towards saturation at higher concentration.21
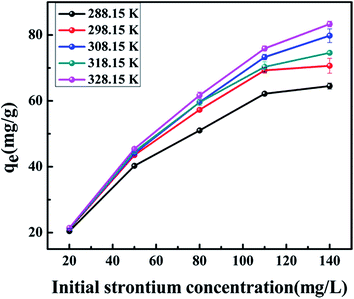 |
| | Fig. 7 Effect of initial strontium concentration on strontium adsorption by the core–shell zeolite@Alg–Ca adsorbent (adsorbent dose: 0.05 g, pH: 4, contact time: 24 h, temperature: 288.15–328.15 K). | |
3.7 The affinity of the adsorbent for strontium
The affinity of the adsorbents for strontium was expressed in terms of the distribution coefficient Kd, using the following equation:22| |
 | (3) |
where C0 and Ce are the initial and equilibrium concentrations of the Sr(II) ions (mg L−1), V is the volume (mL) of the testing solution, and m is the amount of the adsorbent (g) used in the experiment. Values of Kd equal to or above 104 mL g−1 are considered excellent. The core–shell zeolite@Alg–Ca particles show very high Kd values of 3.14 × 104 mL g−1 at 328.15 K, an initial concentration of 20 mg L−1, pH 4, and an adsorbent dose of 0.05 g (shown in Table 6), revealing excellent affinity of the adsorbents for strontium.
3.8 Effect of contact time on adsorption and adsorption kinetics
Fig. 8 shows the effect of contact time on the adsorption amount of strontium on core–shell zeolite@Alg–Ca particles. It was observed that the adsorption amount of Sr(II) ions increased rapidly at the initial stage and then gradually slowed down until it reached equilibrium at about 10 h. The results revealed that, in the beginning, the adsorption of Sr(II) ions mainly occurred on the surface of the adsorbent, so the adsorption proceeds at a high rate.23 After the adsorption active sites on the surface were saturated, the adsorption gradually proceeds into the inner part of the adsorbent via the diffusion of Sr(II) ions into the polymer matrix, leading to adsorption process becoming slower.24
 |
| | Fig. 8 Effect of contact time on strontium adsorption by core–shell zeolite@Alg–Ca adsorbent (initial strontium concentration: 20 mg L−1, pH: 4, temperature: 298.15 K, adsorbent dose: 0.05 g). | |
In order to investigate the mechanism of adsorption of Sr(II) ions on the core–shell zeolite@Alg–Ca particles, different kinetic models including intra-particle diffusion models, pseudo-first-order and pseudo-second-order were used to simulate the experimental data.
The intra-particle diffusion model is expressed as follows:7
where
qt (mg g
−1) is the adsorption capacity at contact time
t and
ki (mol g
−1 min
−0.5) is the diffusion rate constant of step
i (
i = 1, 2, 3) in the adsorption process. The plot of
qt versus t1/2 is shown in
Fig. 9 and the
k1,
k2 and
k3 calculated from the plot are listed in
Table 1.
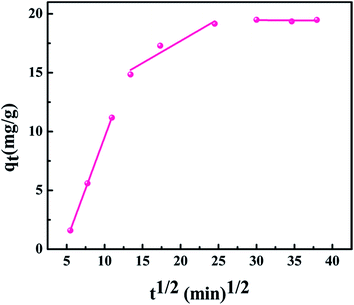 |
| | Fig. 9 Intra-particle diffusion model for the adsorption of strontium by core–shell zeolite@Alg–Ca adsorbent (initial strontium concentration: 20 mg L−1, pH: 4, temperature: 298.15 K, adsorbent dose: 0.05 g). | |
Table 1 Intra-particle diffusion model parameters for the adsorption of strontium by core–shell zeolite@Alg–Ca adsorbent
| Type |
k1 |
R2 |
k2 |
R2 |
k3 |
R2 |
| Intra-particle diffusion model |
1.750 |
0.999 |
0.373 |
0.883 |
−0.0173 |
0.694 |
As shown in Fig. 9, the plot of qt vs. t1/2 consists of three stages and the curves do not pass through the origin point, which indicates that the adsorption processes were not only controlled by surface adsorption, but also controlled by intra-particle diffusion.25 From Table 1, the order of diffusion rate constant value was k1 > k2 > k3, demonstrating that the Sr(II) ions were quickly adsorbed onto the external surface of the core–shell zeolite@Alg–Ca particles at first. Then, the adsorption of strontium on the exterior surface reached equilibrium. Furthermore, strontium transferred from the surface to the core of the adsorbent. Finally, the adsorption of strontium reached final equilibrium.26
The linear and non-linear forms of pseudo-first-order equation are expressed as follows:
| |
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t qe − K1t
| (5) |
The linear and non-linear forms of the pseudo-second-order equation are expressed as follows:
| |
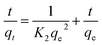 | (7) |
| |
 | (8) |
where
t (min) is the contact time and
qe and
qt (mg g
−1) are the adsorption capacities at equilibrium and contact time
t, respectively.
K1 (min
−1) is the equilibrium constant of the pseudo-first-order model and
K2 (g (mg min)
−1) is the equilibrium constant of the pseudo-second-order model, and they are calculated from the intercept and slope of the plots.
The results are presented in Fig. 10 and Table 2. It was observed that the R2 value for the linear pseudo-second-order model (0.995) was much higher than those of the other models. Moreover, the value of the experimental adsorption capacity (qe,exp) was close to the value of the theoretical adsorption capacity (qe,cal) which was calculated from the linear pseudo-second-order model. These results demonstrated that Sr(II) ion adsorption on the core–shell zeolite@Alg–Ca particles was described by the linear pseudo-second-order model, which indicated that the adsorption process might be chemisorption.27
 |
| | Fig. 10 (a) and (b) Linear pseudo-first-order kinetic and pseudo-second-order kinetic models, (c) and (d) non-linear pseudo-first-order kinetic and pseudo-second-order kinetic models. | |
Table 2 The parameters for pseudo-first-order and pseudo-second-order models for the adsorption of strontium by core–shell zeolite@Alg–Ca adsorbent
| Type |
Pseudo-first-order kinetic |
Pseudo-second-order kinetic |
| qe (mg g−1) |
K1 (min−1) |
R2 |
qe (mg g−1) |
K2 (g (mg min)−1) |
R2 |
| Linear model |
3.06 |
0.00201 |
0.939 |
21.16 |
4.78 × 10−4 |
0.995 |
| Non-linear model |
19.60 |
0.006 |
0.980 |
22.3 |
3.53 |
0.944 |
3.9 Effect of temperature on strontium adsorption and thermodynamic study
The effects of temperature, varying from 288.15 K to 328.15 K, were studied at all the initial strontium concentrations ranging from 20 to 140 mg L−1, and the results are shown in Fig. 11. It was observed that the adsorption capacity of the adsorbent for strontium ions increased as the temperature increased from 288.15 to 328.15 K, which suggested that the adsorption process of strontium ions was and endothermic reaction.
 |
| | Fig. 11 Effect of temperature on strontium adsorption by the core–shell zeolite@Alg–Ca adsorbent (pH: 4, contact time: 10 h, adsorbent dose: 0.05 g, initial strontium concentration: 20–140 mg L−1). | |
To investigate the mechanism involved in the adsorption of strontium, the thermodynamic parameters were calculated by the following equations:
| |
 | (9) |
| |
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 K0
| (10) |
where
K0 is the adsorption equilibrium constant,
R is the gas constant (8.314 J mol
−1 K
−1) and
T is the adsorption temperature (K). Δ
H0 (kJ mol
−1) is the enthalpy change, Δ
S0 (J mol
−1 K
−1) is the entropy change and Δ
G0 (kJ mol
−1) is the Gibbs free energy change. The values of Δ
H0 and Δ
S0 can be obtained from the slope and intercept of a plot of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 vs.
K0 vs. 1/
T and the results are represented in
Table 3 and
Fig. 12.
Table 3 Thermodynamic parameters for the adsorption of strontium by core–shell zeolite@Alg–Ca adsorbent
| T (K) |
ΔH0 (kJ mol−1) |
ΔS0 (J (mol−1 K−1)) |
ΔG0 (kJ mol−1) |
| 288.15 |
15.85 |
79.82 |
−6.819 |
| 298.15 |
15.85 |
79.82 |
−8.250 |
| 308.15 |
15.85 |
79.82 |
−8.745 |
| 318.15 |
15.85 |
79.82 |
−10.13 |
| 328.15 |
15.85 |
79.82 |
−9.786 |
 |
| | Fig. 12 The plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 versus 1/T for strontium adsorption by core–shell zeolite@Alg–Ca adsorbent. K0 versus 1/T for strontium adsorption by core–shell zeolite@Alg–Ca adsorbent. | |
The values of ΔH0 were positive at all temperatures, confirming the endothermic nature of the adsorption process. The negative values of ΔG0 at all temperatures indicated that the adsorption process was spontaneous. In addition, the positive values of ΔS0 suggested an increase in the randomness at the solid-solution surface during the adsorption process of Sr(II) ions by the core–shell zeolite@Alg–Ca particles. This may be due to the increase of mobility in the Sr(II) ions.28
3.10 Adsorption isotherm
The Langmuir and Freundlich isotherms are useful models to describe the interaction of the core–shell zeolite@Alg–Ca particles and Sr(II) ions in this study.
The Langmuir isotherm model assumes monolayer adsorption takes place on a homogenous surface and that there are uniform energies of adsorption onto the surface.29 The linear and non-linear form of Langmuir model can be expressed as follows:30
| |
 | (11) |
| |
 | (12) |
where
qm (mg g
−1) is the maximum adsorption capacity,
Ce (mg L
−1) is the equilibrium concentration of Sr(
II) ions,
qe (mg g
−1) is the adsorption amount of Sr(
II) ions per unit weight of adsorbent at equilibrium, and
KL is the Langmuir constant related to the affinity of binding sites.
The Freundlich model assumes that multilayer adsorption occurs on a heterogeneous surface. The linear and non-linear forms of the Freundlich model can be expressed as follows:31
| |
 | (13) |
| | |
qe = KFCe1/n (non-linear)
| (14) |
where
KF is the Freundlich constant related to the adsorption capacity and 1/
n is the constant related to the adsorption intensity.
The results are presented in Fig. 13 and Table 4. Comparison of the correlation coefficients values (Table 4) revealed that the adsorption of Sr(II) ions onto the core–shell zeolite@Alg–Ca particles was more accurately described by the linear Freundlich model. This indicated that the adsorption of Sr(II) ions took place on the heterogeneous surfaces of the adsorbent. In addition, the values of n were all more than 1 for all temperature tests, which suggested that the adsorption of strontium onto the adsorbent was quite favorable.
 |
| | Fig. 13 (a) and (b) Linear and non-linear Langmuir adsorption isotherm models, (c) and (d) linear and non-linear Freundlich adsorption isotherm models. | |
Table 4 Linear and non-linear of Langmuir and Freundlich models for the adsorption of strontium ion by the core–shell zeolite@Alg–Ca adsorbent
| Type |
T (K) |
Langmuir |
Freundlich |
| qm (mg g−1) |
KL (L mg−1) |
R2 |
KF (mg g−1)(L mg−1)1/n |
n |
R2 |
| Linear model |
288.15 |
60.13 |
0.058 |
0.971 |
17.77 |
3.213 |
0.984 |
| 298.15 |
65.32 |
0.036 |
0.971 |
21.58 |
3.328 |
0.975 |
| 308.15 |
69.11 |
0.033 |
0.960 |
21.86 |
3.074 |
0.994 |
| 318.15 |
65.06 |
0.022 |
0.960 |
24.52 |
3.579 |
0.988 |
| 328.15 |
71.79 |
0.028 |
0.965 |
23.64 |
3.105 |
0.990 |
| Non-linear model |
288.15 |
66.88 |
0.165 |
0.928 |
19.27 |
3.480 |
0.977 |
| 298.15 |
72.99 |
0.229 |
0.933 |
23.97 |
3.732 |
0.963 |
| 308.15 |
83.26 |
0.169 |
0.919 |
23.27 |
3.274 |
0.992 |
| 318.15 |
74.46 |
0.282 |
0.899 |
26.41 |
3.912 |
0.984 |
| 328.15 |
85.40 |
0.204 |
0.922 |
25.42 |
3.354 |
0.990 |
3.11 Effect of co-existing cations
The effect of co-existing cations in nuclear waste such as Na(I) ions, Mg(II) ions, Cs(I) ions, UO2(II) ions, K(I) ions and Ca(II) ions on Sr(II) ions adsorption was investigated. The initial strontium concentration was kept at 20 mg L−1 and the initial concentration of coexisting ions was varied from 0 to 1000 mg L−1 in to assess the effect of co-existing cations, and the results are shown in Fig. 14. The results indicated that Cs(I) ions had no significant effect on the removal of Sr(II) ions at any of the tested concentrations. In addition, the Na(I) ions, Mg(II) ions, UO2(II) ions and K(I) ions also exerted almost no effect on the adsorption of Sr(II) ions in the concentration range of 20–100 mg L−1, although the adsorption capacity of Sr(II) ions decreased with increasing ion concentrations from 100 mg L−1 to 1000 mg L−1. A large amount of co-existing ions in the solution may change the ionic strength of the solution, thus interfering with the Sr(II) adsorption.32 Ca(II) ions co-existing in the solution in the concentration range of 20–1000 mg L−1 also cause a decrease in the uptake of Sr(II) ions, which may be due to Ca(II) ions competing with Sr(II) for alginate active sites in the ion exchange reaction.
 |
| | Fig. 14 Effect of co-existing cations on strontium adsorption by the core–shell zeolite@Alg–Ca particles. | |
3.12 Regeneration and reusability of the adsorbent
Adsorption–desorption experiments of the adsorbent were carried out to evaluate the regeneration and reusability of the core–shell zeolite@Alg–Ca. After equilibrium adsorption, the adsorbent was regenerated by HCl solution at pH 4 for 24 h. The regenerated adsorbent was then used for the subsequent adsorption experiments. The regeneration efficiency of the core–shell zeolite@Alg–Ca was described in Fig. 15. It shown that the three regeneration efficiencies of core–shell zeolite@Alg–Ca after desorption were 91.340%, 76.528% and 62.677%, respectively, indicating good reusability for the adsorption of Sr(II) ions.
 |
| | Fig. 15 Regeneration capacities of HCl (pH 4) solutions for the core–shell zeolite@Alg–Ca adsorbent with strontium. | |
3.13 Characteristics of core–shell zeolite@Alg–Ca particles
3.13.1 SEM-EDX analysis. A digital image and SEM images are displayed in Fig. 16. Fig. 16a shows that a single core–shell zeolite@Alg–Ca particle is spherical, and the core and shell of the particles are presented in different colors. The diameter of the particle was found to be between 1.5 mm and 2 mm. SEM was employed to examine the morphology of the surface and cross section of the zeolite@Alg–Ca particles (Fig. 16b and c). The rough surface of the particle (Fig. 16b) revealed by SEM was favorable for adsorption of Sr(II) ions onto the core–shell zeolite@Alg–Ca particles. The boundary and texture of the shell and core can be observed clearly, as shown in Fig. 16c, which suggested that the particles were created successfully with a shell and core structure.
 |
| | Fig. 16 Digital image of (a) wet core–shell zeolite@Alg–Ca particles. SEM images of (b) the surface (c) cross section of the core–shell zeolite@Alg–Ca particles. | |
The EDX spectra of core–shell zeolite@Alg–Ca particles before and after adsorption are shown in Fig. 17. It could be observed from the EDX spectra of the adsorbent shell (Fig. 17a) that the elemental peaks of C, O, Na, Cl, Ca appeared at 0.277 keV, 0.525 keV, 1.041 keV, 2.816 keV and 3.692 keV, respectively. New elemental peaks of Al and Si in the EDX spectra of the adsorbent core were recorded at 1.487 keV and 1.740 keV (Fig. 17b), suggesting the presence of zeolite in the adsorbent core. After adsorption of Sr(II) ions, a tiny peak of Sr appeared in the EDX spectra for the core and shell of the adsorbent, confirming that the Sr(II) ion was adsorbed onto the core and shell of the core–shell zeolite@Alg–Ca particles.
 |
| | Fig. 17 EDX spectra of (a) the shell of core–shell zeolite@Alg–Ca particles, (b) the core of core–shell zeolite@Alg–Ca particles, (c) the shell of core–shell zeolite@Alg–Ca particles after adsorption, (d) the core of core–shell zeolite@Alg–Ca particles after adsorption. | |
Furthermore, the atomic content percentages obtained by EDX analysis are given in Table 5. It was found that the atomic content of Na in the core of the zeolite@Alg–Ca particles reduced from 2.64% to 2.56%, and Sr appeared in the core of the particles after adsorption with an atomic content of 2.60%, revealing that an exchange occurred between Na(I) ions in the core of the particles and Sr(II) ions in solution. Moreover, the adsorption of Sr(II) ions on the shell of the particles resulted in a decrease of Na and Ca in the shell of the particles and an increase in Sr content. Based on the above analysis, it was concluded that an ion-exchange reaction had occurred between Na(I) ions, Ca(II) ions and Sr(II) ions during the adsorption process.
Table 5 Atomic contents of the adsorbents obtained using EDX
| Sample |
Atomic content (%) |
| Ca |
Na |
Si |
Al |
Sr |
| Core of zeolite@Alg–Ca |
2.64 |
3.24 |
15.64 |
6.57 |
0 |
| Core of zeolite@Alg–Ca particles after adsorption |
2.56 |
1.01 |
17.62 |
7.18 |
2.60 |
| Shell of zeolite@Alg–Ca |
7.97 |
1.23 |
0 |
0 |
0 |
| Shell of zeolite@Alg–Ca particles after adsorption |
3.16 |
0.03 |
0 |
0 |
3.92 |
3.13.2 XPS. In order to analyze the interactions between adsorbates and adsorbents, XPS spectra were obtained and the typical XPS spectra are shown in Fig. 18. When comparing Fig. 18a with b, the wide scan XPS spectra showed that the main elements in the shell of the adsorbent were Na, C, O and Ca, whereas the main elements in the core of the adsorbent were Na, C, O, Ca, Si and Al, confirming that the zeolite existed in the core. After the adsorption of Sr(II) ions, it was obvious that the Na and Ca peaks weakened, whereas a new peak at the binding energy of 134.10 and 134.83 eV appeared, which is assigned to Sr (Fig. 18b and c). It was confirmed that Sr(II) ions were adsorbed on the core and shell of the core–shell zeolite@Alg–Ca particles, and the adsorption mechanism was ion exchange with the Sr(II) ions replacing Ca(II) ions in the carboxyl groups of the alginate and Na(I) ions in the zeolite.
 |
| | Fig. 18 XPS spectrum of (a) the shell of core–shell zeolite@Alg–Ca particles before and after adsorption, (b) the core of core–shell zeolite@Alg–Ca particles before and after adsorption, (c) Sr 3d of the shell and core. | |
3.14 Mechanism analysis of strontium adsorption
The possible mechanism of the formation of alginate gel particles and strontium adsorption is shown in Fig. 19. In accordance with the results from the EDX and XPS studies, we can conjecture that the possible mechanism for the formation of alginate gel particles and strontium adsorption is: (i) an exchange process between Na(I) ions in the alginate and Ca(II) ions in a solution may exist during the preparation process of the particles, leading to the formation of stable and insoluble gel particles of alginate. Ion exchange between Ca(II) ions in solution and the Na(I) ions of alginate occurred in the core and shell, leading to the formation of the core–shell zeolite@Alg–Ca particles (exhibited in Fig. 19). (ii) The Sr(II) ions may be removed by the core–shell zeolite@Alg–Ca particles through ion exchange with the Ca(II) ions or Na(I) ions in the core and shell of the adsorbent. The coordination of the core and shell of the adsorbent removes strontium.
 |
| | Fig. 19 Mechanism of adsorbent formation and strontium adsorption on core–shell zeolite@Alg–Ca particles. | |
The reaction equations are as follows:
| | |
Alg–Ca + Sr2+ → Alg–Sr + Ca2+
| (15) |
| | |
2Alg–Na + Sr2+ → Alg2–Sr + 2Na+
| (16) |
| | |
Na2O·Al2O3·xSiO2·yH2O + Sr2+ → SrO·Al2O3·xSiO2·yH2O + 2Na+
| (17) |
3.15 Comparative study with other adsorbents
Table 6 shows the comparison of various adsorbents for Sr(II) ion adsorption. The results indicate that the core–shell zeolite@Alg–Ca adsorbent has a high adsorption capacity of 83.31 mg g−1 for Sr(II) ions, and very high Kd values of 3.14 × 104 mL g−1, revealing excellent affinity of the adsorbents for strontium. This may be due to the large amounts of Ca(II) ions in the shell which improve adsorption capacity and the zeolite in the core also helps increase the adsorption capacity of the adsorbent.
Table 6 Comparison of various adsorbents for strontium removala
| Adsorbent |
pH |
Capacity (mg g−1) |
Kd (mL g−1) individual |
Reference |
| “—” represented the information was not given in the references. |
| Alginate beads |
7 |
51 |
— |
33 |
| Magnetic chitosan beads |
8.2 |
11.58 |
— |
34 |
| Magnetic graphene oxides |
4 |
14.706 |
— |
35 |
| Magnetically modified zeolite |
— |
36.63 |
— |
36 |
| SbSi |
0.1 M HNO3 |
— |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
22 |
| KSbSi |
0.1 M HNO3 |
— |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
22 |
| H4(TiO)4(SiO4)3·8H2O |
NCAW simulant |
— |
4.48 × 104 |
37 |
| HK3(TiO)4(SiO4)3·4H2O |
NCAW simulant |
— |
2.02 × 104 |
37 |
| Na4Ti9O20·xH2O |
NCAW simulant |
— |
2.35 × 104 |
37 |
| Na2Ti2O3SiO4·2H2O |
NCAW simulant |
— |
2.70 × 104 |
37 |
| Na2Ti2O3SiO4·2H2O |
NCAW simulant |
— |
2.93 × 104 |
37 |
| Cryptomelane-type manganese oxide CRY-1(K) |
4.07 |
— |
>106 |
38 |
| Todorokite-type manganese oxide TOD-1(Mg) |
7.20 |
— |
>106 |
38 |
| Sandia octahedral molecular sieves |
4–6 |
— |
>106 |
39 |
| K2xMnxSn3−xS6 (x = 0.5–0.95) |
1–14 |
77 ± 2 |
≥105 |
40 and 41 |
| K2xMgxSn3−xS6 (x = 0.5–1) |
7 |
86.89 |
2.1 × 104 |
40 and 42 |
| K2xSn4−xS8−x (x = 0.65–1) |
2–12 |
102 ± 5 |
≥105 |
40 and 43 |
| (Me2NH2)4/3(Me3NH)2/3Sn3S7·1.25H2O |
7 |
65.19 |
88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 863 |
40 and 44 |
| Core–shell zeolite@Alg–Ca particles |
4–7 |
83.31 |
3.14 × 104 |
This work |
4. Conclusions
In this study, a novel core–shell zeolite@Alg–Ca particle was successfully prepared and applied for the removal of strontium from aqueous solutions. The synthesized core–shell zeolite@Alg–Ca particles were stable, durable and exhibited an excellent adsorption capacity for Sr(II) ions (88.31 mg g−1). The optimal condition for the synthesis of core–shell zeolite@Alg–Ca particles included a CaCl2 concentration of 1 wt% at a voltage of 1 kV. The optimum adsorption conditions were as follows: contact time of 10 h and a pH of 4–11, which confirmed that the adsorbent can be useful in a wide pH range. The adsorption isotherm was studied using two isotherm models and fit well to the liner Freundlich model. The adsorption kinetics were linear pseudo-second-order. The process of strontium adsorption was spontaneous, endothermic and entropically favorable. The effect of co-existing cations was also investigated and the adsorption capacity of the adsorbent for Sr(II) ions decreased when Na(I) ions, Mg(II) ions, UO2(II) ions and K(I) ions coexisted in the solution in a concentration range of 100–1000 mg L−1. In particular, Ca(II) ions in the concentration range of 20–1000 mg L−1 caused a significant decrease in the uptake of Sr(II) ions. Based on the adsorption–desorption study, the adsorbent could be reused up to three or more cycles for the removal of Sr(II) ions. The removal of strontium was achieved via ion exchange between Ca(II) ions and Na(I) ions in the core and shell of the adsorbent and strontium in the solution.
Acknowledgements
This work was supported by Nuclear Power Development Special item (13zg610301). The authors would like express our sincere thanks to the financial and technological support of Engineering Research Center of Biomass Materials, Ministry of Education, Southwest University of Science and Technology (14tdsc02).
References
- L. Zhang, J. Y. Wei, X. Zhao, F. Z. Li and F. Jiang, Adsorption characteristics of strontium on synthesized antimony silicate, Chem. Eng. J., 2015, 277, 378–387 CrossRef CAS.
- B. Ma, W. S. Shin, S. W. Oh, Y. J. Park and S. J. Choi, Adsorptive removal of Co and Sr ions from aqueous solution by synthetic hydroxyapatite nanoparticles, Sep. Sci. Technol., 2010, 45, 453–462 CrossRef CAS.
- J. L. Wang and C. Chen, Biosorbents for heavy metals removal and their future, Biotechnol. Adv., 2009, 27, 195–226 CrossRef CAS PubMed.
- S. Rao, B. Paul, K. Lai, S. V. Narasimhan and J. Ahmed, Effective removal of cesium and strontium from radioactive wastes using chemical treatment followed by ultra filtration, J. Radioanal. Nucl. Chem., 2000, 246, 413–418 CrossRef CAS.
- D. R. Raut, P. K. Mohapatra and V. K. Manchanda, A highly efficient supported liquid membrane system for selective strontium separation leading to radioactive waste remediation, J. Membr. Sci., 2012, 390, 76–83 CrossRef.
- Y. Sawaki, T. Ohno, M. Tahata, T. Komiya, T. Hirata, S. Maruyama and Y. Li, The ediacaran radiogenic Sr isotope excursion in the doushantuo formation in the three gorges area, South China, Precambrian Res., 2010, 176, 46–64 CrossRef CAS.
- W. Guan, J. M. Pan, H. X. Ou, X. Wang, X. H. Zhou, W. Hu, C. X. Li and X. Y. Wu, Removal of strontium(II) ions by potassium tetratitanate whisker and sodium trititanate whisker from aqueous solution: equilibrium, kinetics and thermodynamics, Chem. Eng. J., 2011, 167, 215–222 CrossRef CAS.
- E. G. Deze, S. K. Papageorgiou, E. P. Favvas and F. K. Katsaros, Porous alginate aerogel beads for effective and rapid heavy metal sorption from aqueous solutions: effect of porosity in Cu2+ and Cd2+ ion sorption, Chem. Eng. J., 2012, 209, 537–546 CrossRef CAS.
- G. Fundueanu, C. Nastruzzi, A. Carpov, J. Desbrieres and M. Rinaudo, Physico-chemical characterization of Ca-alginate microparticles produced with different methods, Biomaterials, 1999, 20, 1427–1435 CrossRef CAS PubMed.
- A. Bhattacharya and B. N. Misra, Grafting: a versatile means to modify polymers: techniques, factors and applications, Prog. Polym. Sci., 2004, 29, 767–814 CrossRef CAS.
- H. Faghihian, M. Moayed, A. Firooz and M. Iravani, Evaluation of a new magnetic zeolite composite for removal of Cs+ and Sr2+ from aqueous solutions: kinetic, equilibrium and thermodynamic studies, C. R. Chim., 2014, 17, 108–117 CrossRef CAS.
- A. K. Vipin, S. Ling and B. Fugetsu, Removal of Cs+ and Sr2+ from water using MWCNT reinforced Zeolite-A beads, Microporous Mesoporous Mater., 2016, 224, 84–88 CrossRef CAS.
- A. M. El-Kamash, Evaluation of zeolite A for the sorptive removal of Cs+ and Sr(II) ions from aqueous solutions using batch and fixed bed column operations, J. Hazard. Mater., 2008, 151, 432–445 CrossRef CAS PubMed.
- S. Lopez-Orozco, A. Inayat, A. Schwab, T. Selvam and W. Schwieger, Zeolitic materials with hierarchical porous structures, Adv. Mater., 2011, 23, 2602–2615 CrossRef CAS PubMed.
- W. Song, B. Y. Gao, X. Xu, L. L. Xing, S. Han, P. J. Duan, W. C. Song and R. B. Jia, Adsorption–desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater, Bioresour. Technol., 2016, 210, 123–130 CrossRef CAS PubMed.
- G. Keçeli, Removal of strontium ions by a crosslinked copolymer containing methacrylic acid functional groups, J. Radioanal. Nucl. Chem., 2006, 268, 211–219 CrossRef.
- Y. J. Tu, C. F. You, Y. R. Chen, C. P. Huang and Y. H. Huang, Application of recycled iron oxide for adsorptive removal of strontium, J. Taiwan Inst. Chem. Eng., 2015, 53, 92–97 CrossRef CAS.
- W. W. Ngah and S. Fatinathan, Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies, J. Environ. Manage., 2010, 91, 958–969 CrossRef CAS PubMed.
- D. H. K. Reddy, K. Seshaiah, A. V. R. Reddy, M. M. Rao and M. C. Wang, Biosorption of Pb2+ from aqueous solutions by Moringa oleifera bark: equilibrium and kinetic studies, J. Hazard. Mater., 2010, 174, 831–838 CrossRef CAS PubMed.
- M. Kumar, B. P. Tripathi and V. K. Shahi, Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater, J. Hazard. Mater., 2009, 172, 1041–1048 CrossRef CAS PubMed.
- Q. S. Zhou, X. Y. Lin, B. Li and X. G. Luo, Fluoride adsorption from aqueous solution by aluminum alginate particles prepared via electrostatic spinning device, Chem. Eng. J., 2014, 256, 306–315 CrossRef CAS.
- T. Möller, R. Harjula, M. Pillinger, A. Dyer, J. Newton, E. Tusa, S. Amin, M. Webb and A. Araya, Uptake of 85Sr, 134Cs and 57Co by antimony silicates doped with Ti4+, Nb5+, Mo6+ and W6+, J. Mater. Chem., 2001, 11, 1526–1532 RSC.
- H. Paudyal, B. Pangeni, K. Inoue, H. Kawakita, K. Ohto, H. Harada and S. Alam, Adsorptive removal of fluoride from aqueous solution using orange waste loaded with multi-valent metal ions, J. Hazard. Mater., 2011, 192, 676–682 CrossRef CAS PubMed.
- G. Zhang, Z. He and W. Xu, A low-cost and high efficient zirconium- modified-Na-attapulgite adsorbent for fluoride removal from aqueous solutions, Chem. Eng. J., 2012, 183, 315–324 CrossRef CAS.
- S. Jagtap, M. K. Yenkie, S. Das and S. Rayalu, Synthesis and characterization of lanthanum impregnated chitosan flakes for fluoride removal in water, Desalination, 2011, 273, 267–275 CrossRef CAS.
- M. T. Yagub, T. K. Sen and H. M. Ang, Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves, Water, Air, Soil Pollut., 2012, 223, 5267–5282 CrossRef CAS.
- J. He, T. S. Siah and J. P. Chen, Performance of an optimized Zr-based nanoparticle-embedded PSF blend hollow fiber membrane in treatment of fluoride contaminated water, Water Res., 2014, 56, 88–97 CrossRef CAS PubMed.
- S. İnan and Y. Altaş, Preparation of zirconium–manganese oxide/polyacrylonitrile (Zr–Mn oxide/PAN) composite spheres and the investigation of Sr(II) sorption by experimental design, Chem. Eng. J., 2011, 168, 1263–1271 CrossRef.
- M. Wang, L. Xu, J. Peng, M. Zhai, J. Li and G. Wei, Adsorption and desorption of Sr(II) ions in the gels based on polysaccharide derivates, J. Hazard. Mater., 2009, 171, 820–826 CrossRef CAS PubMed.
- I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- H. Bradl, 2004 Update Supplement, Adsorption of heavy metal ions on clays, Encyclopedia of Surface and Colloid Science, 2004, vol. 5, p. 35 Search PubMed.
- H. J. Hong, J. Ryu, I. S. Park, T. Ryu, K. S. Chung and B. G. Kim, Investigation of the strontium (Sr(II)) adsorption of an alginate microsphere as a low-cost adsorbent for removal and recovery from seawater, J. Environ. Manage., 2016, 165, 263–270 CrossRef CAS PubMed.
- C. Gok, U. Gerstmann and S. Aytas, Biosorption of radiostrontium by alginate beads: application of isotherm models and thermodynamic studies, J. Radioanal. Nucl. Chem., 2013, 295, 777–788 CrossRef CAS.
- Y. W. Chen and J. L. Wang, Removal of radionuclide Sr(II) ions from aqueous solution using synthesized magnetic chitosan beads, Nucl. Eng. Des., 2012, 242, 445–451 CrossRef CAS.
- D. Li, B. Zhang and F. Xuan, The sequestration of Sr(II) and Cs(I) from aqueous solutions by magnetic graphene oxides, J. Mol. Liq., 2015, 209, 508–514 CrossRef CAS.
- H. Faghihian, M. Moayed, A. Firooz and M. Iravani, Synthesis, characterization, andevaluation
of a ferromagnetically modified natural zeolite composite for removal of Cs+ and Sr2+, Clays Clay Miner., 2013, 61, 193–203 CrossRef CAS.
- P. Sylvester, E. A. Behrens, G. M. Graziano and A. Clearfield, An assessment of inorganic ion-exchange materials for the removal of strontium from simulated Hanford tank wastes, Sep. Sci. Technol., 1999, 34, 1981–1992 CrossRef CAS.
- A. Dyer, M. Pillinger, J. Newton, R. Harjula, T. Möller and S. Amin, Sorption behavior of radionuclides on crystalline synthetic tunnel manganese oxides, Chem. Mater., 2000, 12, 3798–3804 CrossRef CAS.
- M. Nyman, A. Tripathi, J. B. Parise, R. S. Maxwell and T. M. Nenoff, Sandia octahedral molecular sieves (SOMS): structural and property effects of charge-balancing the MIV-substituted (M = Ti, Zr) niobate framework, J. Am. Chem. Soc., 2002, 124, 1704–1713 CrossRef CAS PubMed.
- M. J. Manos and M. G. Kanatzidis, Metal sulfide ion exchangers: superior sorbents for the capture of toxic and nuclear waste-related metal ions, Chem. Sci., 2016, 7, 4804–4824 RSC.
- M. J. Manos, N. Ding and M. G. Kanatzidis, Layered metal sulfides: exceptionally selective agents for radioactive strontium removal, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3696–3699 CrossRef CAS PubMed.
- J. L. Mertz, Z. H. Fard, C. D. Malliakas, M. J. Manos and M. G. Kanatzidis, Selective removal of Cs+, Sr2+, and Ni2+ by K2xMgxSn3−xS6 (x = 0.5–1)(KMS-2) relevant to nuclear waste remediation, Chem. Mater., 2013, 25, 2116–2127 CrossRef CAS.
- D. Sarma, C. D. Malliakas, K. S. Subrahmanyam, S. M. Islam and M. G. Kanatzidis, K2xSn4−xS8−x (x = 0.65–1): a new metal sulfide for rapid and selective removal of Cs+, Sr2+ and UO22+ ions, Chem. Sci., 2016, 7, 1121–1132 RSC.
- X. H. Qi, K. Z. Du, M. L. Feng, J. R. Li, C. F. Du, B. Zhang and X. Y. Huang, A two-dimensionally microporous thiostannate with superior Cs+ and Sr2+ ion-exchange property, J. Mater. Chem. A, 2015, 3, 5665–5673 CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t
qe − K1t
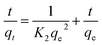




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0
K0
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 vs. 1/T and the results are represented in Table 3 and Fig. 12.
K0 vs. 1/T and the results are represented in Table 3 and Fig. 12.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 versus 1/T for strontium adsorption by core–shell zeolite@Alg–Ca adsorbent.
K0 versus 1/T for strontium adsorption by core–shell zeolite@Alg–Ca adsorbent.







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863
863






