Interfacial synthesis of magnetic PMMA@Fe3O4/Cu3(BTC)2 hollow microspheres through one-pot Pickering emulsion and their application as drug delivery†
Xiaomin Zhu,
Shenping Zhang,
Lihuo Zhang,
Honglai Liu and
Jun Hu*
Key Laboratory for Advanced Materials and Chemistry Department, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China. E-mail: junhu@ecust.edu.cn; Fax: +86-21-64252630; Tel: +86-21-64252630
First published on 13th June 2016
Abstract
In situ interfacial growth of nanoparticles induced by the Pickering emulsion is a novel and convenient method for preparing hybrids/composites with specific tasks. For the first time, magnetic PMMA@Fe3O4/Cu3(BTC)2 hollow microspheres were produced by one-pot Pickering emulsion using Fe3O4 nanoparticle (NP) stabilizer. The Fe3O4 Pickering emulsion provided a large oil/water interface area for the in situ growth of Cu3(BTC)2 nanocrystals and further interfacial polymerization of polymethyl methacrylate (PMMA). The hollow hybrid microspheres showed synergetic multi-functions, including the magnetic separation property caused by Fe3O4 NPs, the reverse thermal expansion by PMMA, and the adsorption enhanced permeation by porous Cu3(BTC)2 NPs. When it was used as the drug carrier for ibuprofen, the good loading capacity and the controllable release duration confirmed its good properties. Moreover, all the realized synergetic advantages further reveled the potential promising applications of the interfacial synthesis by Pickering emulsion method.
Introduction
Pickering emulsions, stabilized by the self-assembly of nanoparticles (NPs) at liquid–liquid interface, constitute a new strategy for encapsulation and transport of drugs in pharmaceutical field. Many amphiphilic NPs such as silica, proteins, gold nanoparticles, bacteria, graphene oxide and carbon have been used as Pickering emulsion stabilizers.1–5 Among various stabilizers, Fe3O4 NP has aroused particular interests because of its outstanding magnetic properties and biocompatibility, which exhibits high performance in magnetic resonance imaging (MRI), targeted drug delivery, protein purification, detection and magnetic damping and etc.6–8 Recently, surface modified Fe3O4 NPs, such as acid-coated,9 polystyrene-brush-coated10 and carbonyl iron11 have been reported as successful stabilizers to prepare magnetic Pickering emulsions.Pickering emulsions are intimately related to the self-assembly of the NPs themselves in the interface of mixture solutions,12–15 therefore the emulsion droplets can be hardly taken out from the solutions because of the poor mechanical stability, which significantly limited their applications. Interfacial polymerization is a promising solution to enhance the mechanical stability of the Pickering emulsion, diverse hollow polymer/NP hybrids have been produced.16–21 In fact, inspired by the well-established mixed matrix membrane separation approach,22 not only the mechanical stability of Pickering emulsions, but also the permeation properties of the emulsion boundary can be enhanced by a good combination of compatible porous NPs and polymers. Recently, Huo et al.23 demonstrated a facile preparation of metal–organic framework (MOF)/polymer microcapsules by assembling ZIF-8 NPs to produce emulsion droplets and followed by an interfacial copolymerization of styrene and divinylbenzene. The combination of small porous NPs and polymer at the interface of microcapsules exhibited good retention for encapsulated dye molecules. This is an inspiration for drug delivery, because the retention and the corresponding release of drug molecules from robust composite colloidosomes could be adjusted by the combination of NPs and polymers. However, the compatibility between polymer and NP stabilizer still remains a great challenge. The most promising strategy for producing well compatible polymer/NP hybrids usually relies on the pre-fabrication of NPs with surface functional modifications, which are usually complicated.
In fact, Pickering emulsion droplets can provide a large liquid–liquid interface for the in situ interfacial growth of NPs. According to our previous work,24,25 MOFs, with oil-soluble organic ligands and water-soluble metal ions26–28 are especially suitable for the interfacial growth at the oil/water interface of emulsions. However, very few works have been reported based on this strategy.29 Herein, we proposed and demonstrated a convenient one-pot Pickering emulsion method to achieve a novel multi-functional hollow microsphere. As illustrated in Scheme 1, we selected Fe3O4 NP as the stabilizer to produce a stable magnetic Pickering emulsion. Then, the ultra-stable hollow microspheres were obtained through a two-step interfacial synthesis, i.e. the in situ growth of Cu3(BTC)2 NPs and the polymerization of methyl methacrylate (PMMA). It was worthy to mention, although all the reactants were added together in one-pot, this complex Pickering emulsion system was still quite stable. The designed microspheres were anticipated to possess multi-functions, including the magnetic separation property caused by Fe3O4 NPs, the reverse thermal expansion by PMMA, and the adsorption enhanced permeation by porous Cu3(BTC)2 NPs. Then, we used them as the drug delivery for ibuprofen to demonstrate the reality of the proposed multi-functions.
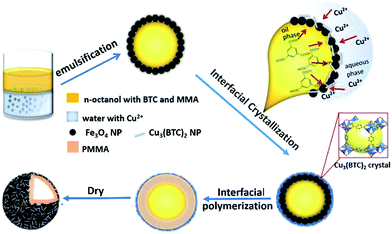 | ||
| Scheme 1 Fabrication of the hollow magnetic-MOF composite through the interfacial growth approach induced by Fe3O4 stabilized Pickering emulsion. | ||
Results and discussion
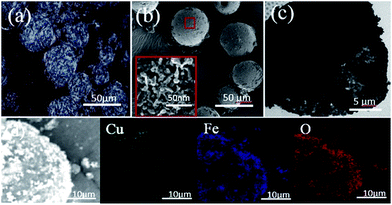
As shown in Scheme 1, the Pickering emulsion system was stabilized by Fe3O4 NPs, where the precursor ligand of MOF Cu3(BTC)2, 1,3,5-benzenetricarboxylic acid (BTC), was dissolved in n-octanol phase, and Cu2+ ion was in water phase. Moreover, the methyl methacrylate (MMA) monomers and 2,2-azobisisobutyro-nitrile (AIBN) initiator were also dissolved in the oil phase. After a strong emulsification at room temperature, the Pickering emulsion droplets, with a diameter about 40–50 μm, were produced and showed a relatively homogeneous size distribution (Fig. S1a, ESI†). Because BTC molecules and Cu2+ ions would diffuse towards the n-octanol/water interface of the emulsion via the free diffusion, by carefully adjusting the synthesis conditions, the interfacial nucleation of Cu3(BTC)2 nanoparticle took place. We found the surface of the Fe3O4 emulsion droplets gradually changed into rough and blue (Fig. S1b, ESI†). Observed by the polarization optical micrograph (POM), the emulsion surface is covered with a shiny crystal shell, in which the crystals are needle-like, uniform-sized, and compactly packed together (Fig. 1a). The crystal structure of the obtained composite was demonstrated by the powder X-ray diffraction (PXRD). Compared with the reference samples of pristine Fe3O4 and Cu3(BTC)2, the presence of both characteristic peaks in the PXRD pattern of the obtained composites (Fig. S2(a), ESI†) confirmed that Cu3(BTC)2 crystals had successfully grew up at the interface of the Fe3O4 emulsion. Because of the confined space effect of the Fe3O4 nanoparticle layer, it was difficult to produce normal 3D octahedral Cu3(BTC)2 crystals, instead, lacking the growth perpendicularly to the interface of the emulsion droplets, the unusual needle-like morphology of crystal Cu3(BTC)2 was obtained. The optical microscope (OM) image of the dried Fe3O4/Cu3(BTC)2 composite (Fig. S3a, ESI†) also revealed the distorted shapes of microspheres with an average size of about 45 μm, but the mechanical stability of the composite was not good enough under the high vacuum analysis condition, some microspheres were broken.To enhance the mechanical strength, the interfacial polymerization was initiated by heating the one-pot Fe3O4/Cu3(BTC)2 composite Pickering emulsion system at 70 °C. n-Octanol is a good solvent for MMA but a poor solvent for PMMA. Therefore, PMMA chains would diffuse into water phase. However, because of the aggregations of Fe3O4/Cu3(BTC)2 at the interface, the diffusion was hold up. As a result, the polymerization of MMA preferred occurring at the interface, and flexible PMMA chains inlayed into the voids between Fe3O4 and Cu3(BTC)2 NPs. Then the stable hybrid microspheres with enough mechanical strength were obtained through the solvent removal. Compared with the reference samples of the pristine PMMA and Cu3(BTC)2, the presence of both characteristic peaks in the Fourier transform infrared spectroscopy (FTIR) spectra of the obtained hybrid microspheres (Fig. S2(b), ESI†) confirmed the successful polymerization of PMMA and formation of Cu3(BTC)2. The FESEM image (Fig. 1b) reveals the perfect retention of the solidified spherical morphology, and from the broken hole of the microsphere, we can confirm its hollow structure. The enlargement of its surface (Fig. 1b insert) shows that many needle-like NPs are densely packed together and embedded in PMMA. The TEM image (Fig. 1c) further reveals its hollow morphology and the shell consists of the aggregations of NPs. The elemental mapping images of Cu, Fe, and O (Fig. 1d) by the energy-dispersive X-ray spectroscopy (EDS) show their spatial distributions on the surface of microspheres, which prove that well mixed Cu3(BTC)2 and Fe3O4 NPs are homogeneously distributed in the entire surface of the hollow microsphere. All these confirmed that we successfully fabricated the hollow hybrid microspheres through the in situ interfacial growth of Cu3(BTC)2 NPs and interfacial polymerization in one-pot Pickering emulsion system. It was found, without the formation of Cu3(BTC)2, the Fe3O4 Pickering emulsion itself was not stable enough to support the interfacial polymerization of MMA to produce the hollow microspheres (Fig. S3b, ESI†), which may be attributed to the poor compatibility between Fe3O4 NPs and PMMA. Therefore, these in-site interfacial grown Cu3(BTC)2 nanocrystals significantly enhanced the stability of the hollow hybrid microspheres because the ligand BTC in Cu3(BTC)2 nanocrystals would exhibit good affinity with PMMA chains.
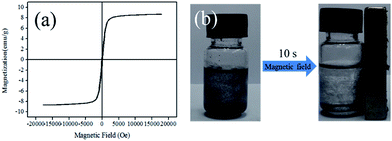
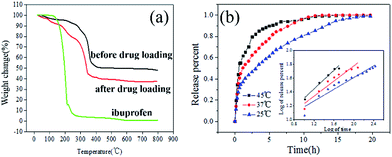
Because of the thermal sensitivity of PMMA, the diameter of hollow PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres increased with increasing the temperature (Fig. 2). The average size of the hollow microspheres swelled from 45 um at 25 °C to about 60–70 um at 50 °C; further increasing the temperature resulted into the distortion of the microspheres, and the average size increased to about 90 um at 70 °C. The thermo-gravimetric analysis (TGA) result of the hollow PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres (Fig. 4a and S4, ESI†) revelled that its thermal stability was as high as about 250 °C, depending much on the decomposition of PMMA. Moreover, magnetic measurement (Fig. 3a) shows that the saturated mass magnetization of PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres is 8.7 emu g−1, with the coercivity of 9.840 Oe and the retentivity of 1.106 emu g−1, suggesting its soft magnetic character. Although the value of the saturated mass magnetization was not very high, the obtained microspheres can quickly respond to the external magnetic field. As shown in Fig. 3b, the microspheres dispersed in n-hexane solution showed a very fast movement to the applied magnetic field (750 Oe) within 10 s, which ensured the efficiency of the magnetic separation.
 | ||
| Fig. 2 OM images of hollow PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres at different temperatures. (a) 25 °C, (b) 50 °C, and (c) 70 °C. | ||
To further demonstrate the multi-functions of the obtained hollow PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres, we took the drug delivery application as an example. Ibuprofen was selected as the drug model because it had been well studied in many drug deliveries. Compared with the TGA curve of hybrid microspheres before loading ibuprofen, there was an extra weight loss in the TGA curve after loading ibuprofen (Fig. 4a), corresponding to the characteristic decomposition of ibuprofen, suggesting hollow PMMA@ Fe3O4/Cu3(BTC)2 hybrid microspheres could successfully act as the drug delivery for loading of ibuprofen. The ibuprofen loading capacity increased with increasing loading temperature and time (Fig. S5, ESI†). Because higher temperature made PMMA chains stretched and the microspheres swelled, resulting larger channels in the microsphere shell for ibuprofen molecules transferring in. After 12 h's loading at 50 °C, the ibuprofen loading capacity in PMMA@Fe3O4/Cu3(BTC)2 microspheres was calculated as high as 250 mg g−1 (sample). Considering the larger molecular mass of Fe3O4 and Cu3(BTC)2, the loading capacity was quite good, higher than most reported loading capacity (Table S1, ESI†). Moreover, after loading ibuprofen, the magnetic hollow hybrid microspheres can be easily separated from n-hexane solution by the magnetic separation (Fig. 3b).
The release profiles of ibuprofen from the hollow PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres (Fig. 4b) show that the release rate can be adjusted by controlling the release temperature. We adopted the Korsmeyer–Peppas kinetic model (eqn (1)) to fit the release data. As listed in Table 1, both the rate constant k and the exponent parameter n increase with the temperature. At 25 °C, n is 0.425 (smaller than 0.45), revealing the Fickian diffusion release, that ibuprofen release depended much on the concentration gradient. Except for the very short initial fast release due to a few ibuprofen molecules adsorbed at the external surface, we can see the release rate is very stable, almost a linear release during the following 20 h, which would be attributed to the synergetic effect of well mixed porous Cu3(BTC)2 NPs and PMMA chains. It should be noted that, although the size of the ibuprofen molecule (0.5 × 1.0 × 0.8 nm) is extremely close to the edge length of the square channels in Cu3(BTC)2 (0.95 nm), the diagonal of the square channels created in Cu3(BTC)2 was determined to be 1.33 nm. Thus the ibuprofen molecule with a flat molecular shape can still be adsorbed inside of Cu3(BTC)2. Similar result was also found in Ferey and coworkers's recent study on MIL-53 (0.86 nm) for controlled drug release, in which ibuprofen loading capacity was as high as 280 mg g−1.30 Therefore, like many small reservoirs for the storage of ibuprofen, the well distributed Cu3(BTC)2 NPs in the PMMA matrix can synergistically exhibit good retention and enhance stable release of ibuprofen. Whereas the drug release mechanism changes into a non-Fickian diffusion when the temperatures increases above 37 °C. As we discussed above, with increasing the temperature, the stretched PMMA chains would induce the expansion of hollow microspheres, making an anomalous diffusion of ibuprofen molecules. The release time is shortened to about 15 h. The higher the temperature, the stronger the influence. At 45 °C, much expanded hollow microspheres as well as the faster diffusion rate of ibuprofen molecule made a much faster release, with an even shorter release time of 7 h. It is possible to control the release performance by adjusting the temperature. Therefore, the encapsulation application of hollow PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres exhibited its good magnetic property, thermo-sensitivity and controllable permeation, which realized our proposed multi-functions and would also be inspiration for many other applications.
| 25 °C | 37 °C | 45 °C | ||
|---|---|---|---|---|
| Korsmeyer–Peppas Mt/M∞ = ktn | n | 0.425 | 0.547 | 0.672 |
| K | 0.328 | 0.454 | 0.684 | |
| R2 | 0.935 | 0.929 | 0.975 |
Experimental
Materials
Fe3O4 magnetic nanoparticles (20 nm, spheroidal) was purchased from Aladdin Industrial Corporation. Copper(II) acetate monohydrate (Cu(NO3)2·H2O, >98%) was purchased from Sinopharm Chemical Reagents Co. Ltd. 1,3,5-Benzenetricarboxylic acid (H3BTC, >99%) was purchased from Sigma Aldrich. N-Octanol (>99%) and methyl methacrylate (MMA, >98%) were purchased from Shanghai LingFeng Chemical Reagent Co., Ltd. 2,2-Azobisisobutyronitrile (AIBN, >98%) was purchased from J&K Scientific. All chemicals were used without further purification.Synthesis of magnetic PMMA@Fe3O4/Cu3(BTC)2 hollow composites
Typically, 0.25 g of Cu(NO3)2·H2O was dissolved in 16 mL deionized water solution (denoted as solution A). Then, 0.1 g of Fe3O4 magnetic nanoparticles was dispersed in the solution A; 0.1 g of 1,3,5-benzenetricarboxylic acid, 2 mL of MMA and 20 mg of AIBN were dissolved in 10 mL n-octanol (denoted as solution B). Two solutions were mixed and emulsified at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to produce the Pickering emulsion stabilized by Fe3O4 NPs. Cu3(BTC)2 crystals grew up at the interface of the droplets when the emulsion was statically placed at the room temperature for 24 h, then, the obtained emulsion was moved into a 70 °C oven for the interfacial polymerization for 10 h. After the filtration and drying at 120 °C under vacuum for 8 h, the PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres were obtained. For comparison, Fe3O4/Cu3(BTC)2 composite microspheres were also produced in the similar way, except for adding of MMA and AIBN.
000 rpm for 5 min to produce the Pickering emulsion stabilized by Fe3O4 NPs. Cu3(BTC)2 crystals grew up at the interface of the droplets when the emulsion was statically placed at the room temperature for 24 h, then, the obtained emulsion was moved into a 70 °C oven for the interfacial polymerization for 10 h. After the filtration and drying at 120 °C under vacuum for 8 h, the PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres were obtained. For comparison, Fe3O4/Cu3(BTC)2 composite microspheres were also produced in the similar way, except for adding of MMA and AIBN.
Characterization
The morphology of samples was characterized by Laser scanning confocal on a Nikon H550S and optical micrograph (OM) on a Nikon A1R, and a Nova NanoS 450 field emission scanning electron microscope (FESEM) operated at 5 kV. Transmission electron microscopy (TEM) images were obtained using a JEM-1400 electron microscope. The Fourier transform infrared (FTIR) spectra were carried out on a Nicolet iS10 FTIR spectrometer using the KBr pellet technique. Powder X-ray diffraction (XRD) measurements were carried out on a D/Max-2550 VB/PC diffractometer (40 kV, 200 mA), using Cu Ka radiation. The thermal stability of hollow microspheres and the amount of ibuprofen loaded was detected using a TGA unit (NETZSCHSTA 499 F3). About 10 mg of the sample was heated from 25 °C to 800 °C with a heating rate of 10 °C min−1 in nitrogen with a flow rate of 40 mL min−1. The elemental analysis and chemical characterization of the samples was characterized on a SEMEDS energy-dispersive X-ray spectroscopy (EDS). Magnetization of the samples were carried out on a Lakeshore 7407 vibration sample magnetometer (VSM). The concentration of ibuprofen was analyzed by ultraviolet spectroscopy (UV) on a Shimadzu UV-2450 at the specific temperature. The absorption wavelength was set at 220.0 nm.Drug loading and release
10 mL ibuprofen (50.0 mg) hexane solution was injected into the sealed container which contains 10 mg PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres. Then the mixtures were stirred at 50 °C for 2, 6, 12 h, respectively, to investigate the loading time influence. Moreover, the loading temperature changed into 25 °C for 12 h, too, to investigate the effect of the temperature. We used TGA to analysis the loading amount of ibuprofen. Before the TGA measurement, hollow microspheres after drug loading were washed by deionized water and ethanol, repeatedly; and were dried at 80 °C in a vacuum oven overnight.The release profiles of ibuprofen were obtained by placing 10 mg hollow hybrid microspheres with ibuprofen loaded into a dialysis bag with an intercept molecular weight of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The dialysis bag was immersed in 100 mL phosphate buffer solution (PBS) (0.01 mol L−1, pH = 7.4) under stirring at 25 °C, 37 °C and 45 °C, respectively. 2 mL solution was collected for the release test at each given time interval, and was returned back immediately after the test.
000. The dialysis bag was immersed in 100 mL phosphate buffer solution (PBS) (0.01 mol L−1, pH = 7.4) under stirring at 25 °C, 37 °C and 45 °C, respectively. 2 mL solution was collected for the release test at each given time interval, and was returned back immediately after the test.
Kinetic study for the drug release
We tried different kinetic models to analyse the release data of ibuprofen. Among them, the comprehensive Korsmeyer–Peppas model (eqn (1)) fitted quite well,| Mt/M∞ = ktn | (1) |
Conclusions
In summary, we reported the convenient synthesis strategy of in situ growth of magnetic hollow PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres induced by one-pot Pickering emulsion. Stabilized by the Fe3O4 NPs, needle-like Cu3(BTC)2 nanocrystals grew up at the interface of the Pickering emulsion droplets, following the in situ interfacial polymerization, the stable hollow PMMA@Fe3O4/Cu3(BTC)2 hybrid microspheres were produced. When the hollow microspheres were used as ibuprofen delivery, they exhibited a good performance in the drug loading, magnetic separation and temperature-depended release, which would have a train of thought in other applications. Moreover, the type of the NPs stabilizer, the resultant NPs, or the polymers can be alternatively changed depending on specific tasks. Therefore, this strategy would have great inspiration for the fabrication of various task-specific composite Pickering emulsions, nanoparticle composites, as well as the hollow microspheres.Acknowledgements
Financial support for this work is provided by the National Basic Research Program of China (2013CB733501), the National Natural Science Foundation of China (No. 91334203, 21376074), Shanghai Municipal Bureau of Quality and Technical Supervision Foundation of China (No. 2015-03), the 111 Project of China (No. B08021), and the project of FP7-PEOPLE-2013-IRSES (PIRSES-GA-2013-612230).Notes and references
- S. Crossley, J. Faria, M. Shen and D. E. Resasco, Science, 2010, 327, 68 CrossRef CAS PubMed.
- W. Zhai, G. Li, P. Yu, L. Yang and L. Mao, J. Phys. Chem. C, 2013, 117(29), 15183–15191 CrossRef CAS.
- K. Zhang, Q. Wang, H. Meng, M. Wang, W. Wu and J. Chen, Particuology, 2014, 14, 12–18 CrossRef CAS.
- G. Yin, Z. Zheng, H. Wang, Q. Du and H. Zhang, J. Colloid Interface Sci., 2013, 394, 192–198 CrossRef CAS PubMed.
- J. Tan, J. Wang, L. Wang, J. Xu and D. J. Sun, J. Colloid Interface Sci., 2011, 359(1), 155–162 CrossRef CAS PubMed.
- C. F. Zhang, B. Wangler, B. Morgenstern, H. Zentgraf, M. Eisenhut, H. Untenecker, R. Kruger, R. Huss, C. Seliger, W. Semmler and F. Kiessling, Langmuir, 2007, 23, 1427–1434 CrossRef CAS PubMed.
- J. L. Corchero and A. Villaverde, Trends Biotechnol., 2009, 27, 468–476 CrossRef CAS PubMed.
- H. W. Gu, K. M. Xu, C. J. Xu and B. Xu, Chem. Commun., 2006, 941–949 RSC.
- Q. Lan, C. Liu, F. Yang, S. Liu, J. Xu and D. J. Sun, J. Colloid Interface Sci., 2007, 310, 260–269 CrossRef CAS PubMed.
- A. Kaiser, T. T. Liu, W. Richtering and A. M. Schmidt, Langmuir, 2009, 25, 7335–7341 CrossRef CAS PubMed.
- S. Melle, M. Lask and G. G. Fuller, Langmuir, 2005, 21, 2158–2162 CrossRef CAS PubMed.
- S. Fujii, M. Okada and T. J. Furuzono, J. Colloid Interface Sci., 2007, 315, 287–296 CrossRef CAS PubMed.
- P. J. Clover and S. A. F. Bon, Chem. Mater., 2007, 19, 1537–1539 CrossRef.
- Y. He, Mater. Lett., 2005, 59, 114–117 CrossRef CAS.
- Q. Qiao, X. K. Tan, L. L. Ji and J. Xue, Synth. Met., 2007, 157, 784–791 CrossRef.
- J. Sun and H. Bi, Mater. Lett., 2012, 81, 48–51 CrossRef CAS.
- Z. Zheng, X. Zheng, H. Wang and Q. Du, ACS Appl. Mater. Interfaces, 2013, 5(16), 7974–7982 Search PubMed.
- L. Zhang, T. Shi, D. Tan, H. Zhou and X. Zhou, Fullerenes, Nanotubes, Carbon Nanostruct., 2014, 22(8), 726–737 CrossRef CAS.
- X. Meng, Y. Guan, Z. Niu and D. Qiu, Langmuir, 2013, 29(7), 2152–2158 CrossRef CAS PubMed.
- J. Li, A. D. Hughes, T. H. Kalantar, I. J. Drake, C. J. Tucker and J. S. Moore, ACS Macro Lett., 2014, 3(10), 976–980 CrossRef CAS.
- A. Schrade, K. Landfester and U. Ziener, Chem. Soc. Rev., 2013, 42, 6823–6839 RSC.
- T. S. Chung, Y. J. Lan, L. Yi and S. Kulprathipanja, Prog. Polym. Sci., 2007, 32, 483–507 CrossRef CAS.
- J. Huo, M. Marcello, A. Garai and D. Bradshaw, Adv. Mater., 2013, 25, 2717–2722 CrossRef CAS PubMed.
- Z. J. Bian, S. P. Zhang, X. M. Zhu, Y. K. Li, H. L. Liu and J. Hu, RSC Adv., 2014, 4, 41902–41909 RSC.
- Z. J. Bian, J. Xu, S. P. Zhang, X. M. Zhu, H. L. Liu and J. Hu, Langmuir, 2015, 31(26), 7410–7417 CrossRef CAS PubMed.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Kim and J. Eddaoudi, Nature, 2003, 423(6941), 705–714 CrossRef CAS PubMed.
- M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112(2), 675–702 CrossRef PubMed.
- M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34(4), 319–330 CrossRef CAS PubMed.
- Y. F. Yang, F. W. Wang, Q. H. Yang, Y. L. Hu, H. Yan, Y. Z. Chen, H. R. Liu, G. Q. Zhang, J. L. Lu and H. L. Jiang, ACS Appl. Mater. Interfaces, 2014, 6, 18163–18171 Search PubMed.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Ferey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11077k |
| This journal is © The Royal Society of Chemistry 2016 |