Efficient chemical synthesis for the analogue of ubiquitin-based probe Ub–AMC with native bioactivity†
Ling
Xu‡
a,
Yang
Xu‡
a,
Qian
Qu
c,
Chao-Jian
Guan
c,
Guo-Chao
Chu
a,
Jing
Shi
*a and
Yi-Ming
Li
*bc
aDepartment of Chemistry, University of Science and Technology of China, Hefei, 230026, China. E-mail: shijing@ustc.edu.cn
bSchool of Biological and Medical Engineering, Hefei University of Technology, Hefei, Anhui 230009, China. E-mail: lym2007@mail.ustc.edu.cn
cThe State Key Laboratory of Medicinal Chemical Biology (NanKai University), China
First published on 9th May 2016
Abstract
The analogue of ubiquitin-based probe ubiquitin–7-amido-4-methylcoumarin (Ub–AMC) was efficiently synthesized through a methyl thioglycolate (MTG) assisted one-pot ligation–desulfurization protocol. To obtain peptide segments with higher yield, norleucine (Nle) was used instead of methionine in the chemical synthesis procedure. Compared with native Ub–AMC, the analogue exhibits well-folded secondary structure and desired bioactivity for studying the DUB enzyme.
Introduction
Ubiquitination, as an important post-translational modification of proteins consisting of 76 amino acid residues, plays an indispensable role in determining cell fate.1–3 The conjugated substrate proteins are mostly degraded or show changed biological properties through the ubiquitin (Ub) mediated procedure by three well known functional enzymes, E1, E2 and E3.4–7 The Ub-activating enzyme E1 initiates the catalytic cascade by forming the thioester linked E1–Ub adduct, which takes place between the C-terminal of Ub and an active thiol of the E1 enzyme with the help of ATP. Subsequently, another thioester linked E2–Ub adduct is generated and Ub is transferred onto the N-terminal or side-chain lysine of the substrate protein through E3 ligase.8,9 As a counteracting mechanism of the ubiquitination system, the attached ubiquitins can be removed by different functional deubiquitinating enzymes (DUBs).10 These DUBs include a large family named cysteine hydrolases that cleave ubiquitin-derived substrates of the general sequence (Ub(1–72)-Leu-Arg-Gly-Gly-X) specifically.11–14 Related studies have proved that the majority of DUBs participated in human major diseases.15–19 To monitor and screen the activity of DUBs, fluorogenic Ub-based probes were developed recently to overcome the insensitivity of HPLC-based test assays.20,21 Among them, Ub–AMC (Ub-7-amino-4-methylcoumarin), which was obtained through the conjugation of AMC at the C-terminal glycine of Ub by protease trypsin, was mostly used due to its high sensitivity and precision.22–24 This type of probe shows gradually enhanced fluorescence under the hydrolysis of DUBs, and therefore, the different activity of DUBs could be monitored.20 Despite the advantage, the procedure of previous strategy for preparing Ub–AMC is still time-consuming and inefficiency.20,25,33,34Recently, the development of chemical protein synthesis makes it possible for acquiring Ub-conjugates in a more convenient and efficient manner.25,26 Some reliable routes towards site-specifically modified Ub-based reagents have been developed. Early approach for acquiring Ub–AMC was achieved by incorporating pseudoproline building blocks and dimethoxybenzyl (DMB) dipeptides into Ub(1–75), and then conjugated with Gly–AMC (Scheme 1a).25 However, the pre-prepared dipeptides unit results in a relatively high cost and a time-consuming process. Another two chemical synthesis approaches including auxiliary-assisted expressed protein liagtion31,32 and hydrazide-based native chemical ligation27–30 both have their own drawbacks,33,34 such as low yield for obtaining recombinant Ub(1–75)–NHNH2 (less than 4 mg L−1) from Ub(1–75)–intein and multiple purification steps of three segments sequential ligation and separated desulfurization.
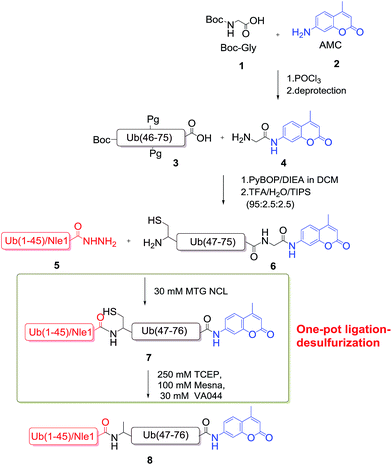
In current work, we present an efficient two-segment ligation strategy for the chemical synthesis of Ub–AMC. Benefiting from our recently reported thiol additive, methyl thioglycolate (MTG),35 this approach could be achieved in an one-pot ligation–desulfurization manner. Moreover, the use of norleucine (Nle) instead of methionine contributes to the higher purity of synthetic peptide segment without oxidation by-product. Bio-activity assay further demonstrated that the Nle substituted Ub–AMC afforded native activity as the fluorogenic substrate of DUB (Scheme 2).
Experiment section
Materials
Dimethyl formamide (DMF), dichloromethane (DCM), CH3OH, CH3CN, EtOAc, piperidine, phenol, tris(2-chloroethyl) phosphate (TCEP), diisopropylethylamine (DIEA), NaNO2, Et2O were purchased from Sinopharm Chemical Reagent Co. Ltd. HCTU, triisopropylsilane (TIPS), 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), trifluoroacetic acid (TFA), methyl thioglycolate (MTG), 1-hydroxybenzotriazole (HOBt), 7-amino-4-methylcoumarin, POCl3 and other common reagents were purchased from Alfa Aesar. All Fmoc-protected amino acids were obtained from GL Biochem (Shanghai) Co., Ltd. 1H NMR, was recorded on a 400 MHz spectrometer and data are reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, coupling constant (Hz)).HPLC
Analytical and Semi preparative HPLC were run on a SHIMADZU (Prominence LC-20AT; Kyoto, Japan) instrument. Analytical columns (Grace Vydac “Peptide C18”, 250 × 4.6 mm, 5 μm particle size, flow rate 1.0 mL min−1, rt) and Semi preparative columns (Grace Vydac “Peptide C18”, 250 × 10 mm, 5 μm particle size, flow rate 4.0 mL min−1) were used for purification. Both analytical and semi-preparative injections were monitored at 214 nm and 254 nm. Solvent A: acetonitrile solution containing 0.08% TFA, solvent B: 0.1% TFA aqueous solution. Water was filtered through 0.22 μm filter paper and sonicated for 25 min.Mass spectrometry
Agilent 6210 Time of Flight Mass Spectrometer was used to measure ESI mass spectra.Preparation of starting materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM = 1
DCM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for 10 min. Then, the resin was filtered off and Fmoc-NHNH2 in DMF (0.15 g/1.5 mL, w/v) was added to the reaction vessel. The mixture was stirred for 1 h to convert the 2-CTC resin into Fmoc-hydrazide resin. Subsequently, the 2-CTC resin was capped with 1.5 mL methanol for 0.5 h and washed with DMF and DCM, respectively. Next, the DMF solution of piperidine (20%, v/v) containing 0.1 M HOBt was used to deprotect Fmoc (2 min + 10 min). The resin was washed again with DMF and DCM. Then the Fmoc-Phe-OH (1 mmol) was coupled using HCTU/DIEA (0.9 mmol/2 mmol) for 1 h. Peptide elongation was performed by following steps of this deprotection/coupling cycle. The last residue was Fmoc-Met-OH or Fmoc-Nle-OH. Once chain assembly was completed, the side-chain deprotection and peptide release was conducted by treatment of reagent B (TFA/phenol/H2O/TIPS = 88
1, v/v) for 10 min. Then, the resin was filtered off and Fmoc-NHNH2 in DMF (0.15 g/1.5 mL, w/v) was added to the reaction vessel. The mixture was stirred for 1 h to convert the 2-CTC resin into Fmoc-hydrazide resin. Subsequently, the 2-CTC resin was capped with 1.5 mL methanol for 0.5 h and washed with DMF and DCM, respectively. Next, the DMF solution of piperidine (20%, v/v) containing 0.1 M HOBt was used to deprotect Fmoc (2 min + 10 min). The resin was washed again with DMF and DCM. Then the Fmoc-Phe-OH (1 mmol) was coupled using HCTU/DIEA (0.9 mmol/2 mmol) for 1 h. Peptide elongation was performed by following steps of this deprotection/coupling cycle. The last residue was Fmoc-Met-OH or Fmoc-Nle-OH. Once chain assembly was completed, the side-chain deprotection and peptide release was conducted by treatment of reagent B (TFA/phenol/H2O/TIPS = 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for 2 h. Finally, two peptide segments, i.e. Ub(1–45)–NHNH2 with N-terminal methionine (1091 mg) or norleucine (1028 mg) were obtained and characterized by HPLC. The Ub(Met1Nle1-45)–NHNH2 crude product was purified by semi-preparative HPLC, obtaining 573.6 mg purified peptide.
2) for 2 h. Finally, two peptide segments, i.e. Ub(1–45)–NHNH2 with N-terminal methionine (1091 mg) or norleucine (1028 mg) were obtained and characterized by HPLC. The Ub(Met1Nle1-45)–NHNH2 crude product was purified by semi-preparative HPLC, obtaining 573.6 mg purified peptide.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM = 1
DCM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). After the reagent was filtered, Fmoc-protected glycine (1 mmol) was resembled on the 2-CTC resin with DIEA (2 mmol) for 1.5 h. Then methanol (4 mL) was added to cap the 2-CTC resin for 0.5 h. The procedures for the removal of Fmoc group and the coupling of amino acid were similar as above mentioned. However, the last amino acid was coupled with Boc-protected cysteine without further removal treatment. After 8 mL solvent (HFIP
1, v/v). After the reagent was filtered, Fmoc-protected glycine (1 mmol) was resembled on the 2-CTC resin with DIEA (2 mmol) for 1.5 h. Then methanol (4 mL) was added to cap the 2-CTC resin for 0.5 h. The procedures for the removal of Fmoc group and the coupling of amino acid were similar as above mentioned. However, the last amino acid was coupled with Boc-protected cysteine without further removal treatment. After 8 mL solvent (HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM = 1
DCM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) was added to cut off the fully protected peptide from the resin, the solution was collected by filtration. The filtrate was condensed by rotary evaporator, then washed and precipitated with cold ether. Through centrifugation (2×, 4000 rpm, 3 min) and air drying, 790 mg (0.13 mmol) protected crude was obtained and it was re-dissolved in 10 mL DCM. The coupling was conducted by treatment of 0.65 mmol Gly–AMC, 0.65 mmol PyBOP and 1.3 mmol DIEA. The solution was stirred overnight at room temperature and then concentrated by evaporation to afford the protected Ub(A46C–76)–AMC. Deprotection was carried out under reagent B (TFA
4, v/v) was added to cut off the fully protected peptide from the resin, the solution was collected by filtration. The filtrate was condensed by rotary evaporator, then washed and precipitated with cold ether. Through centrifugation (2×, 4000 rpm, 3 min) and air drying, 790 mg (0.13 mmol) protected crude was obtained and it was re-dissolved in 10 mL DCM. The coupling was conducted by treatment of 0.65 mmol Gly–AMC, 0.65 mmol PyBOP and 1.3 mmol DIEA. The solution was stirred overnight at room temperature and then concentrated by evaporation to afford the protected Ub(A46C–76)–AMC. Deprotection was carried out under reagent B (TFA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TIPS = 93
TIPS = 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). After precipitation with cold ether, 468 mg crude peptide Ub(A46C–76)–AMC was obtained. The purified product was obtained through semi-preparative HPLC (220 mg).
2). After precipitation with cold ether, 468 mg crude peptide Ub(A46C–76)–AMC was obtained. The purified product was obtained through semi-preparative HPLC (220 mg).
1H NMR of glycine–7-amino-4-methylcoumarin: (400 MHz, D2O) δ 7.16 (d, J = 8.7 Hz, 1H), 7.09 (s, 1H), 6.91 (d, J = 8.6 Hz, 1H), 5.84 (s, 1H), 3.85 (s, 2H), 2.03 (s, 3H).
Results and discussion
We began with the chemical synthesis of peptide segment 5 through a well-developed Fmoc-SPPS (solid-phase peptide synthesis) protocol. Unfortunately, the yield of natural Ub(1–45)–NHNH2 segment with N-terminal methionine is relative low (less than 30% isolated yield). As shown in Fig. 1, although product 5 could be found in HPLC analysis, the major peak (5a) was Met-oxidization by-product. To overcome the shortcoming, we used norleucine instead of methionine for preventing oxidation as previous reports.36–38 As expected, peptide segment Ub(Met1Nle-45)–NHNH2 could be obtained with increased yield (55.8% isolated yield) with only one major peak in HPLC analysis. More importantly, compared with natural peptide, norleucine derivatives can be easily purified by using semi-preparative HPLC chromatograph on a large scale. | ||
| Fig. 1 The chemical synthesis of Ub(M1Nle-45)–NHNH2 segment (observed mass (M + H): 5109.9, calculated mass (M): 5108.6). | ||
To prepare segment 6, we first obtained compound Gly–AMC (4) through an easy-to-obtained route from materials Boc-Gly (1) and 7-amino-4-methyl coumarin (2).33 Another peptide segment Ub(46C–75) can be easily synthesized by air-bath heating Fmoc-SPPS method rather than divided into two parts.39 With two fragments in hands, ligation was performed at room temperature to form the protected Ub(46C–76)–AMC. We found that the most part of crude peptide Ub(46C–75) was converted to product 6 after overnight reaction. After final de-protection and purification, AMC modified peptide 6 can be obtained with high purity (estimated to 47% isolated yield) (Fig. 2).
 | ||
| Fig. 2 (a) HPLC monitoring of the ligation between Ub(46C–76)–AMC and Gly–AMC. (b) HPLC and ESI-MS analysis of purified Ub(46C–76)–AMC. | ||
Then, we began to assemble the full-length Ub–AMC by using hydrazide-based NCL procedure. Because Ala-45 was first mutated to Cys for ligation, it was necessary to obtain protein with native sequence through cysteine desulfurization. However, we found that the previous two-step ligation–desulfurization procedure was not indeed efficiency mainly because of the dual purification steps, which result in relative low yield and time-consuming process. Inspired by our recent developed MTG (methyl thioglycolate) mediated one-pot straightforward ligation–desulfurization protocol,35 we performed these sequential process in an one-pot manner with only final purification step. In details, the peptide segment 5 was first converted to Ub(1–45)–MTG (5c) after MTG thiol (30 equiv.) was added. Then, the ligation was started after the addition of segment 6 (1 equiv., pH 6.8) by using hydrazide-based NCL protocol. After 9 h reaction, new product peak 7 was formed companied with the disappearance of original material (segment 5c and 6, estimated to 90% HPLC yield, Fig. 3). Then we started to carry out the desulfurization reaction without purification by adding 250 mM TCEP, 100 mM MESNa and 30 mM VA-044 into the ligation buffer (final pH 6.5, 37 °C). About 12 h, the reaction was completely finished to give the final product 8 (70% HPLC yield). SDS-PAGE and ESI-MS analysis confirmed the correct weight of Ub–AMC (Fig. 3b and c).
 | ||
| Fig. 3 (a) HPLC monitoring of the ligation with two segments. (b) SDS-PAGE for the native Ub and Ub–AMC. (c) ESI-MS for the product 7 and 8. | ||
Before determine the bioactivity of synthetic probe, spontaneous folding of Ub–AMC was conducted by directly dissolving lyophilized ubiquitin in aqueous buffer or ddH2O. Then, refolded product was characterized by CD spectra compared with expressed ubiquitin, which confirmed the well-folded secondary structure in ddH2O (1 mg mL−1). Because DUBs can promote the release of strongly fluorescent AMC moiety from Ub–AMC, the bioactivity of Nle substitute Ub–AMC was tested by monitoring the fluorescence change upon the hydrolysis mediated by one kind of DUBs (USP7). We found that the fluorescence showed rapidly increasing when USP7 was added (Ub–AMC 300 nM and USP7 100 pM), while it did not cause any change in the absence of USP7. The results indicate that the synthetic Ub–AMC performed well as active fluorescent probe for studying the activity of DUB enzymes (Fig. 4).
 | ||
| Fig. 4 (a) CD spectra of synthetic Ub–AMC and wild-type Ub. (b) Enzymatic activity of Ub–AMC with and without USP7 enzyme. | ||
Conclusion
In summary, we developed an efficient method to synthesize an analogue of ubiquitin-based probe Ub–AMC relying on two-segment hydrazides based NCL coupled with one-pot ligation–desulfurization. Another key to this technical advance is the use of Nle instead of Met residue in the process of the synthesis for peptide segment. Nle substituted peptide features high purity due to less oxidation by-product. The synthetic probe was verified to afford the well-folded structure and satisfied bioactivity for DUB study. We hope that the utility of this simply operational approach would contribute to the chemical synthesis of other important ubiquitin-based probe.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21372058, 21572043 and 21572214), and the State Key Laboratory of Medicinal Chemical Biology.Notes and references
- A. Hershko and A. Ciechanover, Annu. Rev. Biochem., 1998, 67, 425–479 CrossRef CAS PubMed.
- O. Kerscher, R. Felberbaum and M. Hochstrasser, Annu. Rev. Cell Dev. Biol., 2006, 22, 159–180 CrossRef CAS PubMed.
- M. Hochstrasser, Nature, 2009, 458, 422–429 CrossRef CAS PubMed.
- D. Popovic, D. Vucic and I. Dikic, Nat. Med., 2014, 20, 1242–1253 CrossRef CAS PubMed.
- M. Hejjaoui, M. Haj-Yahya, K. S. A. Kumar, A. Brik and H. A. Lashuel, Angew. Chem., Int. Ed., 2011, 50, 405–409 CrossRef CAS PubMed.
- S. P. Jackson and D. Durocher, Mol. Cell, 2013, 49, 795–807 CrossRef CAS PubMed.
- D. Vucic, V. M. Dixit and I. E. Wertz, Nat. Rev. Mol. Cell Biol., 2011, 12, 439–452 CrossRef CAS PubMed.
- L. Spasser and A. Brik, Angew. Chem., Int. Ed., 2012, 51, 6840–6862 CrossRef CAS PubMed.
- D. Komander and M. Rape, Annu. Rev. Biochem., 2012, 81, 203–229 CrossRef CAS PubMed.
- F. E. Reyes-Turcu and K. Wilkinson, Chem. Rev., 2009, 109, 1495–1508 CrossRef CAS PubMed.
- K. D. Wilkinson, Annu. Rev. Nutr., 1995, 15, 161–189 CrossRef CAS PubMed.
- A. L. Goldberg and W. N. Mitch, N. Engl. J. Med., 1997, 335, 1897–1905 Search PubMed.
- T. Hadari, J. V. B. Warms, I. A. Rose and A. Hershko, J. Biol. Chem., 1992, 267, 719–727 CAS.
- K. D. Wilkinson, V. L. Tashayev, L. B. O'Conner, C. N. Larsen, E. Kasperek and C. M. Pickart, Biochemistry, 1995, 34, 14535–14546 CrossRef CAS PubMed.
- R. C. Conaway, C. S. Brower and J. W. Conaway, Science, 2002, 296, 1254–1258 CrossRef CAS PubMed.
- R. K. McGinty, J. Kim, C. Chatterjee, R. G. Roeder and T. W. Muir, Nature, 2009, 453, 812–816 CrossRef PubMed.
- S. M. B. Nijman, M. P. A. Luna-Vargas, A. Velds, T. R. Brummelkamp, M. G. Dirac, T. K. Sixma and R. Bermards, Cell, 2005, 123, 773–786 CrossRef CAS PubMed.
- D. Komander, M. J. Clague and S. Urbe, Nat. Rev. Mol. Cell Biol., 2009, 10, 550–563 CrossRef CAS PubMed.
- T. T. Huang and A. D. Andrea, Nat. Rev. Mol. Cell Biol., 2006, 7, 323–334 CrossRef CAS PubMed.
- L. C. Dang, F. D. Melandri and R. L. Stein, Biochemistry, 1998, 37, 1868–1879 CrossRef CAS PubMed.
- C. Rivard and M. Bazzaro, Methods Mol. Biol., 2015, 1249, 193–200 CAS.
- F. E. Reyesturcu, K. H. Ventii and K. D. Wilkinson, Annu. Rev. Biochem., 2009, 78, 363–397 CrossRef CAS PubMed.
- D. S. Leggett, J. Hanna, A. Borodovsky, B. Crosas, M. Schmidt, R. T. Baker, T. Walz, H. Ploegh and D. Finley, Mol. Cell, 2002, 10, 495–507 CrossRef CAS PubMed.
- M. Hu, P. Li, M. Li, W. Y. Li, T. T. Yao, J. W. Wu, W. Gu, R. E. Cohen and Y. G. Shi, Cell, 2002, 111, 1041–1054 CrossRef CAS PubMed.
- F. E. Oualid, R. Merkx, R. Ekkebus, D. S. Hameed, J. J. Smit, A. de Jong, H. Hilkmann, T. K. Sixma and H. Ovaa, Angew. Chem., Int. Ed., 2010, 49, 10149–10153 CrossRef PubMed.
- Y. C. Huang and L. Liu, Sci. China: Chem., 2015, 58, 1779 CrossRef CAS.
- P. E. Dawson, T. W. Muir, I. Clark-Lewis and S. B. H. Kent, Science, 1994, 266, 776–779 CAS.
- J. S. Zheng, S. Tang, Y. C. Huang and L. Liu, Acc. Chem. Res., 2013, 46, 2475–2484 CrossRef CAS PubMed.
- G. M. Fang, Y. M. Li, F. Shen, Y. C. Huang, J. B. Li, Y. Lin, H. K. Cui and L. Liu, Angew. Chem., Int. Ed., 2011, 50, 7645–7649 CrossRef CAS PubMed.
- J. S. Zheng, S. Tang, Y. K. Qi, Z. P. Wang and L. Liu, Nat. Protoc., 2013, 8, 2483–2495 CrossRef CAS PubMed.
- T. W. Muir, D. Sondhi and P. A. Cole, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 6705 CrossRef CAS.
- K. Severinov and T. W. Muir, J. Biol. Chem., 1998, 273, 16205 CrossRef CAS PubMed.
- Y. T. Li, J. Liang, J. B. Li, G. M. Fang, Y. C. Huang and L. Liu, J. Pept. Sci., 2014, 20, 102–107 CrossRef CAS PubMed.
- J. Liang, G. M. Fang, X. L. Huang, Z. Q. Mei, J. Li, C. L. Tian and L. Liu, Sci. China: Chem., 2013, 56, 1301–1306 CrossRef CAS.
- Y. C. Huang, C. C. Chen, S. Gao, Y. H. Wang, H. Xiao, F. Wang, C. L. Tian and Y. M. Li, Chem. Eur. J., 2016 DOI:10.1002/chem.201600101.
- M. Miller, J. Schneider, B. K. Sathyanarayana, M. V. Toth, G. R. Marshall, L. Clawson, L. Selk, S. B. H. Kent and A. Wlodawer, Science, 1989, 246, 1149–1152 CAS.
- G. M. Fang, J. X. Wang and L. Liu, Angew. Chem., Int. Ed., 2012, 51, 10347 CrossRef CAS PubMed.
- S. Tang, Y. Y. Si, Z. P. Wang, K. R. Mei, X. Chen, J. Y. Cheng, J. S. Zheng and L. Liu, Angew. Chem., Int. Ed., 2015, 54, 5713 CrossRef CAS PubMed.
- Y. C. Huang, C. J. Guan, X. L. Tan, C. C. Chen, Q. X. Guo and Y. M. Li, Org. Biomol. Chem., 2015, 13, 1500–1506 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11019c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |