One-pot synthesis of sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of lead(ii)†
Abstract
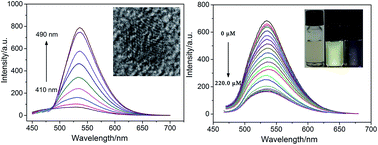
A novel one-pot synthesis of sulfur-doped graphene quantum dots (S-GQDs) was proposed based on water-phase molecular fusion with 1,3,6-trinitropyrene, Na2S, and NaOH in a hydrothermal process. A 75% yield was obtained and mass production of S-GQDs with high crystallinity was possible. The prepared S-GQDs gave a stable yellow-green emission within a wide pH range of 2.0–11.0 and exhibited excitation-independent photoluminescence behaviors. As investigated by atomic force microscopy (AFM), the synthesized S-GQDs possessed monolayer-graphene thickness. As illustrated by transmission electron microscopy (TEM), the synthesized S-GQDs exhibited high crystallinity and uniform size (∼3 nm). Successful doping of S atoms in graphene quantum dot lattices was proven by X-ray photoelectron spectroscopy (XPS) characterization. Compared with GQDs, the S-GQDs had drastically changed surface chemistry and showed a selective and sensitive response to Pb2+. Ions such as Na+, K+, Cu2+, Ca2+, Mg2+, Zn2+, Fe3+, Ni2+, Co2+, Cd2+ have no effect on the fluorescence of S-GQDs. Based on the fluorescence quenching of S-GQDs by Pb2+ in water, a facile and direct fluorescence sensor for Pb2+ detection was developed. Under the optimized conditions, the linear response ranged from 0.1 to 140.0 μM with a detection limit of 0.03 μM.

 Please wait while we load your content...
Please wait while we load your content...