Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spectroscopic differentiation between monomeric and aggregated forms of BODIPY dyes: effect of 1,1-dichloroethane†
Marlius Castilloa,
Sangram L. Rautb,
Sarah Pricea,
Ilkay Boraab,
Laramie P. Jamesona,
Changling Qiuc,
Kevin A. Schugc,
Zygmunt Gryczynski*b and
Sergei V. Dzyuba*a
aDepartment of Chemistry and Biochemistry, Texas Christian University, Fort Worth, TX 76129, USA. E-mail: s.dzyuba@tcu.edu; Tel: +1 817 257 6218
bDepartment of Physics and Astronomy, Texas Christian University, Fort Worth, TX 76129, USA. E-mail: z.gryczynski@tcu.edu; Tel: +1 817 257 4309
cDepartment of Chemistry and Biochemistry, The University of Texas at Arlington, Arlington, TX 76019, USA
First published on 14th July 2016
Abstract
1,1-Dichloroethane induces a monomer–aggregate equilibrium of common BODIPY dyes in organic solvents at low μM dye concentrations, thus providing an opportunity to study monomeric, aggregate-free forms of BODIPY dyes.
Fluorophore assemblies are of significance due to their unique photophysical properties related to their optical and electronic responses,1–4 as well as their applications in analytical and material sciences.5–13
BODIPY dyes are a widely used class of fluorescent probes, which attract continuous interest because their photophysical properties could be tuned via fairly straightforward synthetic transformations.14–17 J- and H-aggregates of BODIPY dyes were noted in a number of studies; however, these cases primarily involved aggregation in the solid or gel states or in organic solvent-water mixtures.18–24 The aggregated forms of BODIPY dyes, especially in aqueous environments, tend to exhibit a red-shifted transition at ca. 600 nm. A few cases of BODIPY H-aggregates were reported, and in some instances the H-aggregates of BODIPY dyes were found to be non-emissive.18–20 Notably, in these cases, the BODIPY dyes possessed specific moieties that induced a particular aggregation state.25 On the other hand, in organic solvents, BODIPY dyes are known to be monomeric, especially at low μM concentration ranges, as confirmed by the presence of a single peak in the emission spectra.18,24,26,27 Here, we present an unusual case of spectroscopically distinct monomeric and aggregated forms of BODIPY in a chlorinated organic solvent.
Building on our interest in developing molecular viscometers based on BODIPY scaffolds,28–30 we examined the spectroscopic properties of BODIPY dimer 1 (Fig. 1) in various organic solvents, and found that both absorption and emission spectra of 1 in 1,2-DCE were distinctly different from those in other solvents, such as EtOH, DMSO, THF, dioxane, CH3CN, and CHCl3 (Fig. 1A). Specifically, notable hypsochromic shifts were observed in both the absorption (from ca. 540 to 515 nm) and emission (from ca. 560 to 535 nm) spectra (Fig. 1B and C).
 | ||
| Fig. 1 Structure of dye 1; (A) absorption (left) and emission (right) spectra of [1] = 1.0 μM in various organic solvents; concentration dependent absorption (B) and emission (C) spectra of dye 1 in 1,2-DCE (ACROS/for spectroscopy; Table S1†), arrows above the spectra show the direction of the spectral shift upon increasing dye concentration. Insets: changes in the absorption and emission maxima, and changes in the absorption an emission intensity at specified wavelengths as a function of dye concentration. | ||
To verify the aggregate formation, we measured fluorescence lifetime and anisotropy as a function of dye 1 concentration (Tables S2 and S3, Fig. S1†). A decrease in the average fluorescence lifetime was accompanied by an increase in the steady state anisotropy over 0.1–10 μM. In addition, the anisotropy data showed that at low concentrations (i.e., 0.1 and 0.2 μM), only the monomeric form of 1 was present, as two components were sufficient to fit the anisotropy decay. The fast correlation time (93 ps) was attributed to the rotation of the BODIPY units around the diyne moiety, while the slower correlation time (541 ps) was attributed to tumbling of dye 1 in solution. At higher concentrations (≥0.5 μM) two components were no longer sufficient to fit the anisotropy decay, and the appearance of a third component (rinf) was consistent with the presence of large (i.e., aggregated) species, whose correlation time was much longer than the lifetime of the probe, thus requiring the use of rinf (Table S3†).31
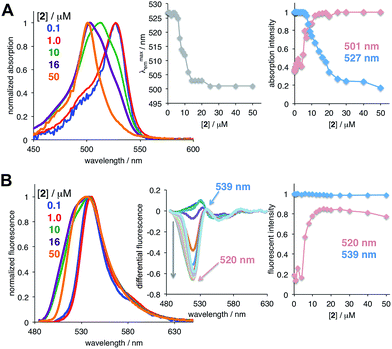
Next, in order to determine whether the observed monomer-aggregate behavior was specific to 1, we evaluated the concentration dependent spectroscopic responses of several common BODIPY dyes (see ESI†), which did not possess any aggregation-inducing moieties (Fig. 2 and S2†).32 In all cases, hypsochromic shifts in both absorption and emission spectra were observed. Because substantial changes in both absorbance and emission spectra were noted for 2, this dye was chosen for all subsequent studies as a model compound.
 | ||
| Fig. 2 Concentration dependent absorption (A) and emission (B) spectra of dye 2 in 1,2-DCE (ACROS/for spectroscopy; Table S1†); arrows above the spectra show the direction of the spectral shift upon increasing dye concentration. Insets: changes in the absorption and emission maxima, and changes in the absorption an emission intensity at specified wavelengths as a function of dye concentration. | ||
Due to the unprecedented ability of 1,2-DCE to induce H-aggregate formation, we screened several samples of 1,2-DCE from various vendors (Fig. S3†), and unexpectedly found that the differentiation between monomeric and aggregated forms of BODIPY 2 was only observed in 1,2-DCE from ACROS (ACROS/for spectroscopy, Table S1†). Subjecting 1,2-DCE samples (ACROS/ACS-spectrograde and ACROS/for spectroscopy, Table S1†) to various treatments (purging with nitrogen, washing with water and 1 N HCl) did not change the initial observation with this sample of 1,2-DCE, i.e., no differentiation between the monomeric and aggregated forms of BODIPY was observed. In an attempt to clarify this discrepancy, we subjected several samples to GC-VUV (gas chromatography with vacuum ultraviolet detector) analysis,33 which established that 1,1-DCE was the only impurity present in ACROS/for spectroscopy and not ACROS/ACS-spectrograde 1,2-DCE (Fig. S4;† vinyl chloride was not considered due to high volatility).
To prove that 1,1-DCE was capable of inducing the formation of monomeric 2, a small amount of 1,1-DCE was added to several organic solvents (Fig. 3). In the case of EtOH and CH3CN (solvents in which no spectroscopic differentiation between monomeric and aggregated forms of BODIPY dyes was noted) a bathochromic shift was observed upon addition of 1,1-DCE (Fig. 3A and C). This confirmed that 1,1-DCE was responsible for providing the spectroscopic distinction between the monomeric and aggregated forms of BODIPY. Arguably, the inability of 1,1-DCE to induce a spectral shift in CHCl3 and DMSO (Fig. 3B and D) could be attributed to the stronger solvation of the dye in these solvents. Notably, 1,1-DCE samples were purchased from different vendors (Aldrich and TCI,‡ Table S1†) and similar results were observed. Importantly, when concentration dependent absorption spectra were acquired in 1,1-DCE and two spectroscopic grades of 1,2-DCE (Fig. S5†), a hypsochromic shift from 529 nm to 502 nm was only noted in 1,2-DCE (ACROS/for spectroscopy), a single absorption maximum was noted at 502 nm and at ca. 528 nm in 1,2-DCE (ACROS/ACS-spectrograde) and 1,1-DCE, respectively. Furthermore, the anisotropy measurements confirmed the absence of the aggregated form of dye 2 in 1,1-DCE upto 10 μM, whereas in 1,2-DCE the aggregated form of 2 was noted even at 1.0 μM (Fig. S6, Table S3†).
 | ||
| Fig. 3 Normalized absorption spectra of dye 2 (0.1 μM) in various solvents ((A) EtOH; (B) CHCl3; (C) CH3CN; (D) DMSO) in the absence (blue) and in the presence (red) of 5% (v/v) of 1,1-DCE. | ||
Next, to further characterize the behavior of 2 in 1,1-DCE (Fig. 4, S7 and S8, and Table S4†), more detailed spectroscopic measurements were carried out, even though due to a relatively high cost,§ 1,1-DCE is more likely to be used as an additive rather than a solvent.¶ The concentration dependent spectra only started to exhibit the apparent hypsochromic shift above 8 μM (Fig. 4A). Emission spectra on the other hand did not exhibit significant changes upon increasing the concentration of dye 2 (Fig. 4B). However, differential spectra (Fig. 4B, inset) unambiguously demonstrated that the emission of the higher energy species increased with increasing concentrations of the dye.
Furthermore, the full width half maxima (FWHM) as a function of increasing dye's concentration (Fig. S7†) indicated a gradual transition from a monomeric species to an aggregate.
In conclusion, we showed that it is possible to spectroscopically distinguish the monomeric and aggregated forms of BODIPY dyes in 1,1-DCE. Notably, small quantities of 1,1-DCE could be used to modify the aggregate–monomer equilibrium in several organic solvents. These results could provide a novel approach toward the superior evaluation and characterization of fluorophores and their assemblies, in general, and BODIPY-based dyes, in particular. Therefore, additive-induced disaggregation could be a valuable approach towards modulating the supramolecular structure of fluorophore assemblies, which could have far reaching applications in a variety of fields. Further studies on the behavior of other BODIPY dyes in 1,1-DCE as well as the mechanism behind the 1,1-DCE-induced differentiation between the monomeric and aggregated forms of BODIPY dyes are in progress in our laboratories and will be reported in due course.
Acknowledgements
This work was partially supported by NSF (CBET-1403226 to SVD) and NIH (R21EB017985 and RO1EB12003 to ZG). KAS and CQ would like to thank VUV Analytics, Inc. for research and instrumental support.Notes and references
- K. Nakata, T. Kobayashi and E. Tokunaga, Phys. Chem. Chem. Phys., 2011, 13, 17756–18867 RSC.
- Y. Xiong, H. Tang, J. Zhang, Z. Y. Wang, J. Campo, W. Wenseleers and E. Goovaerts, Chem. Mater., 2008, 20, 7465–7473 CrossRef CAS.
- Q. Meng, C. Zhang, Y. Zhang, Y. Zhang, L. Yao, Y. Liao and Z. Dong, Appl. Phys. Lett., 2015, 107, 043103 CrossRef.
- M. Baghgar and M. D. Barnes, ACS Nano, 2015, 9, 7105–7112 CrossRef CAS PubMed.
- S. Basak, N. Nandi, A. Baral and A. Banerjee, Chem. Commun., 2015, 51, 780–783 RSC.
- Y. Hu, L. Meng and Q. Lu, Langmuir, 2014, 30, 4458–4464 CrossRef CAS PubMed.
- L. I. Markova, V. L. Malinovski, L. D. Patsenker and R. Haener, Chem. Commun., 2013, 49, 5298–5300 RSC.
- A. Sarbu, L. Biniek, J.-M. Guenet, P. J. Mesini and M. Brinkmann, J. Mater. Chem. C, 2015, 3, 1235–1242 RSC.
- A. Diac, D. Demeter, M. Allain, I. Grosu and J. Roncali, Chem.–Eur. J., 2015, 21, 1598–1608 CrossRef CAS PubMed.
- S. Ogi, T. Fukui, M. L. Jue, M. Takeuchi and K. Sugiyasu, Angew. Chem., Int. Ed., 2014, 53, 14363–14367 CrossRef CAS PubMed.
- J.-H. Fuhrhop, Langmuir, 2014, 30, 1–12 CrossRef CAS PubMed.
- F. Fennel, S. Wolter, Z. Xie, P.-A. Ploetz, O. Kuehn, F. Wuerthner and S. Lochnrunner, J. Am. Chem. Soc., 2013, 135, 18722–18725 CrossRef CAS PubMed.
- M. Vybornyi, A. V. Rudnev, S. M. Langenegger, T. Wandlowski, G. Calzaferri and R. Haener, Angew. Chem., Int. Ed., 2013, 52, 11488–11493 CrossRef CAS PubMed.
- N. Boens, B. Verbelen and W. Dehaen, Eur. J. Org. Chem., 2015, 6577–6595 CrossRef CAS.
- A. Bessette and G. S. Hanan, Chem. Soc. Rev., 2014, 43, 3342–3405 RSC.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed.
- D. Tleugabulova, Z. Zhang and J. D. Brennan, J. Phys. Chem. B, 2002, 106, 13133–13138 CrossRef CAS.
- F. Bergrstöm, I. Mikhalyov, P. Hägglöf, R. Wortmann, T. Ny and L. B.-Å. Jahansson, J. Am. Chem. Soc., 2002, 124, 196–204 CrossRef.
- T. T. Vu, M. Dvorko, E. Y. Schmidt, J.-F. Audibert, P. Retailleau, B. A. Trofimov, R. B. Pansu, G. Clavier and R. Meallet-Renault, J. Phys. Chem. C, 2013, 117, 5373–5385 CAS.
- S. Choi, J. Bouffard and Y. Kim, Chem. Sci., 2014, 5, 751–755 RSC.
- L. Yang, G. Fan, X. Ren, L. Zhao, J. Wang and Z. Chen, Phys. Chem. Chem. Phys., 2015, 17, 9167–9172 RSC.
- C. F. A. Gomez-Duran, R. Hu, G. Feng, T. Li, F. Bu, M. Arseneault, B. Liu, E. Peña-Cabrera and B. Z. Tang, ACS Appl. Mater. Interfaces, 2015, 7, 15168–15176 CAS.
- N. K. Allampally, A. Florian, M. J. Mayoral, C. Rest, V. Stepanenko and G. Fernández, Chem.–Eur. J., 2014, 20, 10669–10678 CrossRef CAS PubMed.
- For some recent examples see: (a) G. Gupta, A. Das, N. B. Kikhil, T. Kim, J. Y. Ryu, J. Lee, N. Mandal and C. Y. Lee, Chem. Commun., 2016, 52, 4272–4277 Search PubMed; (b) Z. Zhu, J. Quin, X. Zhao, W. Qin, R. Hu, H. Zhang, D. Li, Z. Xu, B. Z. Tang and S. He, ACS Nano, 2016, 10, 588–597 CrossRef CAS PubMed; (c) E. Sen, K. Meral and S. Atilgan, Chem.–Eur. J., 2016, 22, 736–745 CrossRef CAS PubMed; (d) H. Ma, Z. Zhang, Y. Jin, L. Zha, C. Qi, H. Cao, Z. Yang, Z. Yang and Z. Lei, RSC Adv., 2015, 5, 87157–87167 RSC.
- J. Bañuelos Prieto, F. López Arbeloa, T. Arbeloa, S. Salleres, F. Amat-Guerri, M. Liras and I. López Arbeloa, J. Phys. Chem. A, 2008, 112, 10816–10822 CrossRef PubMed.
- J. Bañuelos Prieto, F. López Arbeloa, V. Martínez Martínez, T. Arbeloa López and I. López Arbeloa, J. Phys. Chem. A, 2004, 108, 5503–5508 CrossRef.
- J. D. Kimball, S. Raut, L. P. Jameson, N. W. Smith, Z. Gryczynski and S. V. Dzyuba, RSC Adv., 2015, 5, 19508–19511 RSC.
- S. Raut, J. Kimball, R. Fudala, H. Doan, B. Maliwal, N. Sabnis, A. Lacko, I. Gryczynski, S. V. Dzyuba and Z. Gryczynski, Phys. Chem. Chem. Phys., 2014, 16, 27037–27042 RSC.
- S. L. Raut, J. D. Kimball, R. Fudala, I. Bora, R. Chib, H. Jaafari, M. K. Castillo, N. W. Smith, I. Gryczynski, S. V. Dzyuba and Z. Gryczynski, Phys. Chem. Chem. Phys., 2016, 18, 4535–4540 RSC.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, 2009 Search PubMed.
- L. P. Jameson and S. V. Dzyuba, Beilstein J. Org. Chem., 2013, 9, 786–790 CrossRef CAS PubMed.
- K. A. Schug, I. Sawicki, D. D. Carlton Jr, H. Fan, H. M. McNair, J. P. Nimmo, P. Kroll, J. Smuts, P. Walsh and D. A. Harrison, Anal. Chem., 2014, 86, 8329–8335 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional spectroscopic characterization of BODIPY dyes in various solvents, and GC-VUV traces. See DOI: 10.1039/c6ra10833d |
| ‡ 1,1-DCE sample from TCI is stabilized with CH3NO2 (Table S3). To rule out the effect of CH3NO2, both absorption and emission of dye 2 were acquired ([2] = 0.1–10 μM); no concentration-dependent hypsochromic shift was observed; thus the behaviour of dye 2 in CH3NO2 was similar to that found for other organic solvents, such as DMSO, THF, etc., (see Fig. 1A). |
| § Aldrich (order #: 36967): $35.40/1 g; TCI (order #: D0363): $81.00/25 g. |
| ¶ For a preliminary spectroscopic characterization of other BODIPY dyes in 1,1-DCE see Table S6. |
| This journal is © The Royal Society of Chemistry 2016 |