Biosynthesis, isolation, and structural characterization of ε-poly-l-lysine produced by Streptomyces sp. DES20†
Abstract
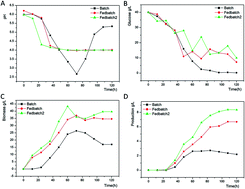
ε-Poly-L-lysine (ε-PL) is an unusual, naturally occurring homo-polyamide made of 25–35 L-lysines with antimicrobial activities. ε-PL is widely used in food and medical fields because of its high levels of safety and biodegradability. In our studies, the effects of different fermentation methods on ε-PL biosynthesis by Streptomyces sp. DES20 were determined. Without any control, 2.637 g L−1 ε-PL was produced in a 5 L fermentation tank in 120 h. When feeding glucose and ammonium sulfate during fermentation, the production yield increased to 6.687 g L−1. After adding 2 g L−1 sodium citrate, the ε-PL yield further increased to 8.351 g L−1 at the end of fermentation. The weak acid anion-exchange resin FPC3500 was used to separate and extract ε-PL from the fermentation liquid. ε-PL was eluted with NH4OH with 85.40% purity at a recovery rate of 80.99%. Whereas through hydrochloric acid elution, the recovery rate of ε-PL increased to 89.23% with 95.26% purity. The degree of polymerization was identified as 26–33 using MALDI-TOF-MS. The ε-PL structure was characterized by ultraviolet spectroscopy, infrared spectroscopy, 1H NMR, 13C NMR, and HMBC. ε-PL exhibits significant antimicrobial activities against four different kinds of bacteria.

 Please wait while we load your content...
Please wait while we load your content...