Role of surfactant and micelle promoted mild, green, highly efficient and sustainable approach for construction of novel fused pyrimidines at room temperature in water†
Pramod K. Sahu*ab
aSchool of Studies in Chemistry, Jiwaji University, Gwalior-474011, Madhya Pradesh, India. E-mail: sahu.chemistry@gmail.com; researchdata6@gmail.com
bDepartment of Industrial Chemistry, Jiwaji University, Gwalior-474011, Madhya Pradesh, India
First published on 20th June 2016
Abstract
A facile, efficient and green protocol for surfactant catalyzed synthesis of fused pyrimidines in water at room temperature was developed for the first time. The influence of the sodium lauryl sulphate (SLS) micelles and their different concentrations on reactivity was studied. It was found that best yield was obtained with 10 mol% catalyst loading with minimum time as compare to other surfactants and catalysts were used. This procedure resulted in a general and environmentally benign protocol has advantage of better efficiency of catalyst, excellent yield, short reaction time and easy to work up.
Introduction
Scaffold decoration of bioactive molecules represents one of the most vibrant research areas in organic chemistry and has a rich history within the realm of fragment-based drug design. Designing the new drugs is based on the development of hybrid molecules by combining different pharmacophore fragments in a single structure, which may lead to compounds with interesting biological profile. As pathogenic bacteria continuously evolve the mechanism of resistance to currently used antibacterial, so the discovery of novel and potent antibacterial drugs is the best way to overcome bacterial resistance and to develop effective therapies.1 Multicomponent reactions (MCRs) are of increasing significance in organic and medicinal chemistry. Multicomponent reactions have been refined in recent years into a powerful and useful tool in synthetic chemistry. Such processes enable the rapid elaboration of complex structures in a highly efficient, higher yield than almost any sequential synthesis of the same target, a single purification step, time and energy saving, low expenditures, easy adaptation to combinatorial synthesis and modular manner. In addition, the implementation of several transformations in a single manipulation is highly compatible with the goals of sustainable and “green” chemistry.2–8Pyrimidine and its derivatives have been studied for several years because of their chemical and biological significance. They have been reported as anti-viral, anti-tumor, anti-inflammatory, antihypertensive activities,9–11 calcium channel modulators12 and antimicrobial agents.13–15 Numerous heterocyclic systems fused with pyrimidines are known for their important biological activities.16 Some chromeno pyrimidine derivatives show antiplatelet and antithrombotic activities.17
The ‘greening’ of global chemical processes has became a major issue in the chemical industry.18 As a consequence, in recent year much effort has been directed toward the use of water as solvent for organic reactions.19–26 The development of simple and efficient chemical processes or methodologies for the synthesis of biologically active compounds in water is one of the major challenges for chemists, although water is a safe, very cheap, readily available, and environmentally benign solvent.27 Recently, many researchers have been synthesized different fused pyrimidines in water.28 Unfortunately, many of these reported methods suffer from one or the other limitations such as drastic reaction conditions, long reaction time with poor yield, side products formation, strong oxidizing agents, use of toxic reagents, use of expensive catalyst, more catalyst loading, and tedious workup procedure. Although today's environmental consciousness imposes the use of water as a solvent on both industrial and academic chemists, organic solvents are still used instead of water for mainly two reasons. First, most organic substances are insoluble in water, and as a result, water does not function as a reaction medium. Second, many reactive substrates, reagents, and catalysts are decomposed or deactivated by water. Some of these problems were solved with the discovery of surfactant combined catalysts by Kobayashi et al.29 Therefore, three-component reaction which exploits different surfactants as catalyst in water could reveal an ideal methodology, provided that the catalyst shows high catalytic activity in water and remove all drawbacks of reported methods.
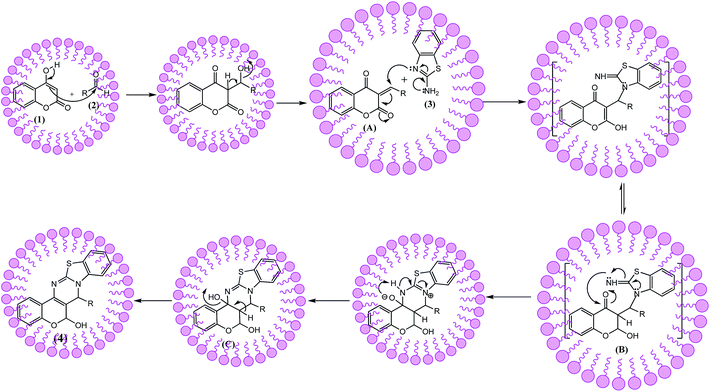
As part of our continuing efforts on the development of new routes for the synthesis of heterocyclic30 herein, we wish to report surfactant miceller catalyzed synthesis of fused pyrimidine by one-pot three component reaction of 4-hydroxy coumarin, aldehydes and urea/thiourea, 3-amino-1,2,4-traizole, or 2-amino benzothiazole in water at room temperature (Scheme 1).
Results and discussion
Three component reaction starting from benzaldehyde, 4-hydroxy coumarin and 2-amino benzothiazole has proved to be a facile method for preparation of chromeno[4,3-d]benzothiazolo[3,2-a]pyrimidin-6(7H)-one (4). To optimize the reaction conditions, first of all we studied the effect of different solvents using SLS (Table 1). Table 1 showed that among all solvents, reaction was well tolerated in water with significant yield isolated in comparable with short reaction time (Table 1, entry 1). That's why water was chosen as solvent for further studies and all other catalysts have used in aqueous medium.From the viewpoint of today's environmental consciousness, however, it is desirable to avoid the use of harmful organic solvents. Therefore, we next initiated investigations to develop a new system for surfactant catalyzed reactions in water without using organic solvents. The main drawback in the use of water (low solubility of most organic substances in water) could be overcome by using surfactants, which solubilize organic materials or form emulsions with them in water. To address this solubility issue, therefore, we planned to use surfactants hopefully small amounts of them for the surfactant catalyzed reactions in water.
To optimize the effect of reaction time on the yield of target product, a model reaction was carried out at different time using 4-hydroxy coumarin (2 mmol), benzaldehyde (2 mmol) and 2-amino benzothiazole (2 mmol) in water using surfactant at room temperature. The reaction time 5 h was found to be optimum time (Fig. 1). Further increasing the reaction time did not increase yield. Highest yield (from 30–91%) of product was obtained at 5 h of reaction time.
Yield of product was dependent on the reaction time in the presence of surfactant as catalyst in water. When the loading of catalyst was not enough, the maximum yield could not be reached. To avoid this kind of problem, an optimum amount of catalyst loading had to be investigated. For this study, synthesis of chromeno[4,3-d]benzothiazolo[3,2-a]pyrimidin-6(7H)-one (4) carried out using 4-hydroxy coumarin (2 mmol), benzaldehyde (2 mmol) and 2-amino benzothiazole (2 mmol) in the presence of different amount of catalyst (2, 5, 8, 10 and 15 mol%) respectively (Table 2). Highest yield was obtained with 10 mol% of SLS as catalyst. The catalyst loading 10 mol% was found to be the optimal quantity. Other surfactants such as SDS and TBAB have also been used as catalyst with same mol% but give lower yield as compared to SLS (Table 2, entries 6 and 7). That's why we have used other catalysts which were also give poor yield as compared to SLS (entries 8–12). So, further we have extended our work with SLS (10 mol%) as catalyst.
| Entry | Catalysts | Time (h) | Yielda (%) |
|---|---|---|---|
| a Isolated yield. | |||
| 1 | Sodium lauryl sulphate (2 mol%) | 7.0 | 52 |
| 2 | Sodium lauryl sulphate (5 mol%) | 6.0 | 78 |
| 3 | Sodium lauryl sulphate (8 mol%) | 5.5 | 86 |
| 4 | Sodium lauryl sulphate (10 mol%) | 5.0 | 91 |
| 5 | Sodium lauryl sulphate (15 mol%) | 5.0 | 91 |
| 6 | Sodium dodesyl sulphate (10 mol%) | 5.0 | 81 |
| 7 | TBAB (10 mol%) | 5.0 | 76 |
| 8 | KCl (10 mol%) | 8.0 | 55 |
| 9 | Mg(NO3)2 (10 mol%) | 9.0 | 51 |
| 10 | p-Toluene sulphonic acid (10 mol%) | 8.0 | 49 |
| 11 | CaCl2 (10 mol%) | 8.5 | 41 |
| 12 | LiBr (10 mol%) | 7.5 | 59 |
After optimization of reaction conditions the reaction was carried out with various aromatic aldehydes, 4-hydroxy coumarin and 2-aminobenzothiazole or urea/thiourea or 3-amino-1,2,4-triazole to afford chromeno[4,3-d]benzothiazolo[3,2-a]pyrimidin-6(7H)-one (4), 3,4-dihydro-1H-chromeno[4,3-d]pyrimidine-2,5-dione (6) and dihydro-6H-chromeno[4,3-d][1,2,4]triazolo[1,5-a]pyrimidin-6-one (8) derivatives respectively. Catalytic activity of SLS has been explored for the synthesis of these derivatives at room temperature in aqueous medium, results are incorporated in Table 3. Table 3 revealed that reaction proceeded efficiently with para substituted aldehydes with good yield whereas ortho and meta substituted aldehydes gave the slightly lower yield comparatively to para substituted aldehydes.
| R | R1 | R2 | Product | Yielda (%) | Time (h) |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| –C6H5 | H | H | 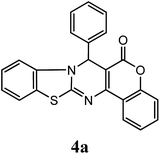 |
95 | 5.0 |
–C6H5–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
H | H |  |
85 | 4.5 |
| 4-Cl–C6H5 | H | H |  |
86 | 4.0 |
| 4 N(CH3)2–C6H5 | H | H | 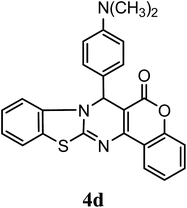 |
94 | 4.0 |
| 3-OH–C6H5 | H | H |  |
93 | 4.0 |
| 4-OCH3–C6H5 | H | H |  |
91 | 4.0 |
| 4-CH3–C6H5 | H | H |  |
94 | 4.5 |
| 4-NO2–C6H5 | H | H | 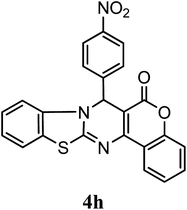 |
86 | 5.0 |
| 2-CH3–C6H5 | H | H | 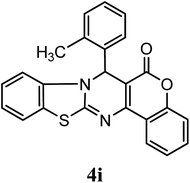 |
91 | 5.0 |
| 2-OCH3–C5H6 | H | H |  |
90 | 5.0 |
| 4-OH–C6H5 | H | H | 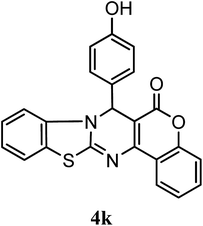 |
91 | 5.0 |
| 4-Cl–C6H5 | Me | H | 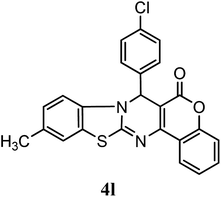 |
85 | 4.5 |
| –C6H5 | NO2 | H |  |
81 | 3.5 |
| 4-CH3–C6H5 | H | Cl |  |
87 | 4.0 |
| 4-Br–C6H5 | Me | Cl | 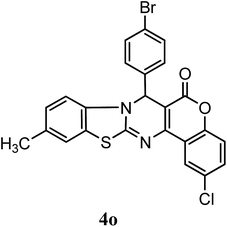 |
88 | 4.0 |
| C6H5 | — | — |  |
95 | 5.0 |
| 4-Cl–C6H5 | — | — | 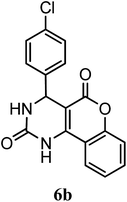 |
93 | 5.0 |
| C6H5 | — | — |  |
91 | 5.0 |
| 4-N(CH3)2–C6H5 | — | — | 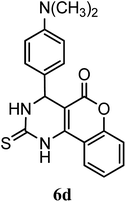 |
93 | 5.0 |
| 2-OH–C6H5 | — | — |  |
81 | 5.0 |
Role of surfactant
The catalytic effect of the micellar solution of may be attributed to the hydrophobic nature of organic substrates. Formation of emulsion droplets takes place in water in the presence of surfactant and substrate molecules. It is suggested that most of the organic substrates are concentrated in these spherical droplets, which act as a hydrophobic reaction sites and results in an increase in the effective concentration of the organic reactants. In micellar solution, organic substrates are pushed away from water molecules towards the hydrophobic core of micelle droplets thus inducing efficient collisions between organic substrates which eventually enhance the reaction rate and result in rapid reactions in water.29,31Mechanism and role of aqueous medium
We speculate that in the presence of organic substrates, SLS molecules form stable colloidal particles in which the surfactant moiety of the SLS surrounds the organic substrates and the counter cations are attracted to the surface of the particles through electrostatic interactions between the anionic surfactant molecules. Although each sodium cation is hydrated by several water molecules, they can be readily replaced by a substrate because of the high exchange rate of sodium for substitution of inner sphere water ligands.32 The substrates to be activated move to the interface from the organic phase, coordinate to the cations, and then react with nucleophilic substances there. The hydrophobic interior of the micelles swiftly excludes the water molecules generated during the reaction, thus shifting the equilibrium towards the desired product that ultimately leads to an increase in the reaction yield.33,34According to results found above, SLS worked well only in water. A comparable study of different solvents in the three component reaction revealed that the reaction in water was faster. In the SLS-catalyzed reactions stated in this article, most of the reaction mixtures became turbid, and formation of these colloidal dispersions is a characteristic feature for the present reaction system. Thus, we have tried to observe the SLS-induced colloidal particles. This observation implies the formation of micelle or micelle like colloidal aggregates, analyzing an aliquot of the reaction mixture, we observed that the spherical particles were clearly formed which is supported by literature (Fig. 2).35–39
A plausible mechanism for the synthesis of chromeno[4,3-d]benzothiazolo[3,2-a]pyrimidin-6(7H)-one derivatives (4) is depicted in Scheme 2. In the beginning, Knoevenagel condensation of the 4-hydroxycoumarin (1) and aldehyde (2) in the presence of SLS produces intermediate. This is followed by Michael addition of 2-aminobenzothiazole (3) to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of intermediate (A) and form intermediate (B) through tautomerization. Then, an intramolecular cyclic condensation between the amino and the carbonyl groups of the Michael adduct B occurs to afford intermediate (C), which afford the desired compounds (4) on dehydration.
C bond of intermediate (A) and form intermediate (B) through tautomerization. Then, an intramolecular cyclic condensation between the amino and the carbonyl groups of the Michael adduct B occurs to afford intermediate (C), which afford the desired compounds (4) on dehydration.
Thus, the micelle intervention consists probably of the concentration and disposition of the chemical species where the exterior coat of the SDS micelle structure would strongly bind to all the reactants (precursors and intermediaries) as well as the final product according to its structural features and electrostatic interactions. Therefore, our proposed scheme is explained in terms of the anionic SDS micelle nature and the concentration effect (hydrophobic interaction between reactants and aqueous medium).40,41
Materials and methods
Experimental
The 1H NMR spectra were measured using BRUKER AVANCE II 400 NMR spectrometer with tetramethylsilane as an internal standard at 20–25 °C; data for 1H NMR are reported as follows: chemical shift (ppm), integration, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet, and br, broad), coupling constant (Hz). IR spectra were recorded by SHIMADZU; IR spectrometer of sample dispersed in KBr pellet or Nujol is reported in terms of frequency of absorption (cm−1). E-Merck pre-coated TLC plates and RANKEM silica gel G were used for preparative thin-layer chromatography. Melting points were determined in open capillaries and are uncorrected. AR grade of 4-hydroxy coumarin, aldehydes, urea, thiourea, 3-amino-1,2,4-triazole, SLS and other catalysts were purchased from Himedia Laboratory Ltd., Mumbai, India. 2-Amino benzothiazole was purchased from Sigma Aldrich and used without further purification.One-pot three-component reaction
Characterization data
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.78 (d, 1H,
CH), 6.78 (d, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.10–7.45 (m, 8H, Ar-H), 7.56 (t, 2H, J = 7.5 Hz, Ar-H), 7.63 (m, 3H, Ar-H); 13C NMR (125 MHz, DMSO d6): 65, 101, 102, 104, 111, 115, 116, 117, 123, 124, 126, 127, 128, 129, 131, 132, 137, 143, 152, 153, 160, 161, 163, 164, 165; ESI-MS: m/z calculated for C25H16N2O2S 408.47 found [M + H]+ 409.
CH), 7.10–7.45 (m, 8H, Ar-H), 7.56 (t, 2H, J = 7.5 Hz, Ar-H), 7.63 (m, 3H, Ar-H); 13C NMR (125 MHz, DMSO d6): 65, 101, 102, 104, 111, 115, 116, 117, 123, 124, 126, 127, 128, 129, 131, 132, 137, 143, 152, 153, 160, 161, 163, 164, 165; ESI-MS: m/z calculated for C25H16N2O2S 408.47 found [M + H]+ 409.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 10.38 (s, 1H, OH); 13C NMR (125 MHz, DMSO d6): 36, 102, 116, 116, 118, 119, 123, 125, 128, 129, 130, 133, 135, 143, 153, 158, 158; ESI-MS: m/z calculated for C18H12N4O3 332.3 found [M]+ 332.3.
CH), 10.38 (s, 1H, OH); 13C NMR (125 MHz, DMSO d6): 36, 102, 116, 116, 118, 119, 123, 125, 128, 129, 130, 133, 135, 143, 153, 158, 158; ESI-MS: m/z calculated for C18H12N4O3 332.3 found [M]+ 332.3.Acknowledgements
We are grateful thanks to SAIF Punjab University Chandigarh, India spectral analytical data.References
- H. Mitsuya, R. Yarchoan and S. Broder, Science, 1990, 249, 1533 CAS.
- C. O. Kappe, Curr. Opin. Chem. Biol., 2002, 6, 314 CrossRef CAS PubMed.
- V. Nair, C. Rajesh, A. Vinod, U. S. Bindu, A. R. Streekenth, S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899 CrossRef CAS PubMed.
- D. J. Ramon and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef CAS PubMed.
- A. A. Domling, Chem. Rev., 2006, 106, 17 CrossRef PubMed.
- A. Domling and I. Ugi, Angew. Chem., 2000, 112, 3300 CrossRef.
- L. F. Tietze and A. Modi, Med. Res. Rev., 2000, 20, 304 CrossRef CAS PubMed.
- J. Zhu, Eur. J. Org. Chem., 2003, 1133 CrossRef CAS.
- C. O. Kappe, Tetrahedron, 1993, 49, 6937 CrossRef CAS.
- K. S. Atwal, G. C. Rovnyak, B. C. O”Reilly and J. Schwartz, J. Org. Chem., 1989, 54, 5898 CrossRef CAS.
- (a) K. S. Atwal, B. N. Swanson, S. E. Unger, D. M. Floyd, S. Moreland, A. Hedberg and B. C. O’reilly, J. Med. Chem., 1991, 34, 806 CrossRef CAS PubMed; (b) G. C. Rovnyak, K. S. Atwal, A. hedberg, S. D. Kimball, S. Moreland, J. Z. Gougoutas, B. C. O'Reillly, J. Schwartz and M. F. Malley, J. Med. Chem., 1992, 35, 3254 CrossRef CAS PubMed.
- (a) G. C. Rovnyak, S. D. Kimbal, B. Beyer, G. Cucinotta, J. D. Dimarco, J. Gougoutas, A. Hedberg, M. Malley, J. P. MaCaethy, R. Zhang and S. Mereland, J. Med. Chem., 1995, 38, 119 CrossRef CAS PubMed; (b) C. O. Kappe, W. M. F. Fabian and M. A. Semons, Tetrahedron, 1997, 53, 2803 CrossRef CAS.
- K. R. Lanjewar, A. M. Rahatgaonkar, M. S. Chorghade and B. D. Saraf, Ind. J. Chem., 2009, 48, 1732 Search PubMed.
- (a) M. M. Heravi, L. Ranjwar, F. Deriknand, B. Alimadadi and H. A. Oskooie, Mol. Diversity, 2008, 12, 181 CrossRef CAS PubMed; (b) S. Chang, J. S. Ji and L. Yu, J. Chin. Chem. Soc., 2008, 55, 292 CrossRef; (c) N. K. Shah, M. P. Patel and R. G. Patel, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 2704 CrossRef CAS.
- R. Kumar, S. Malik and R. Chamdra, Ind. J. Chem., 2009, 48, 718 Search PubMed.
- J. Kempson, W. J. Pitts, J. Barbosa, J. Guo, O. Omotoso, A. Watson, K. Stebbins, G. C. Starling, J. H. Dodd, J. C. Barrish, R. Felix and K. Fischer, Bioorg. Med. Chem. Lett., 2005, 15, 1829 CrossRef CAS PubMed.
- O. Bruno, C. Brullo, S. Schenone, F. Bondavalli, A. Ranise, M. Tognolini, M. Impicciatore, V. Ballabeni and E. Barocelli, Bioorg. Med. Chem., 2006, 14, 121 CrossRef CAS PubMed.
- (a) R. T. Baker and W. Tumas, Science, 1999, 284, 1477 CrossRef CAS; (b) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed; (c) J. Haggin, Chem. Eng. News, 1994, 72, 22 Search PubMed; (d) E. M. Kirschner, Chem. Eng. News, 1994, 72, 13 Search PubMed; (e) D. L. Illman, Chem. Eng. News, 1994, 72, 22 Search PubMed.
- N. Azizi, A. K. Amiri, R. Baghi, M. Bolourtchian and M. M. Hashemi, Monatsh. Chem., 2009, 140, 1471 CrossRef CAS.
- P. A. Grieco, Organic synthesis in water, Blackie Academic and Professional, 1998 Search PubMed.
- C. J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS.
- C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed.
- C. J. Li and T. H. Chan, Organic reaction in aqueous media, Wiley New York, 1997 Search PubMed.
- M. C. Pirrung and K. D. Sarma, J. Am. Chem. Soc., 2004, 126, 444 CrossRef CAS PubMed.
- A. Shaabani, A. Rahmati, A. H. Rezayan, M. Darvishi, Z. Badri and A. Sarvari, QSAR Comb. Sci., 2007, 26, 973 CAS.
- W. Wei, C. C. K. Keh, C. J. Li and R. S. Varma, Clean Technol. Environ. Policy, 2004, 6, 250 CrossRef CAS.
- (a) P. T. Anastas and T. C. Williamson, Green Chemistry ACS Symposium Series 626, American Chemical Society, Washington, DC, 1996 Search PubMed; (b) P. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- (a) D. Prajapati and M. Gohain, Beilstein J. Org. Chem., 2006, 2, 1 CrossRef PubMed; (b) F. Nemati and R. Saeedirad, Chin. Chem. Lett., 2013, 24, 370 CrossRef CAS; (c) G. K. Verma, K. Raghuvanshi, R. Kumar and M. S. Singh, Tetrahedron Lett., 2012, 53, 399 CrossRef CAS; (d) A. Motamedi, E. Sattari, P. Mirzaei, M. Armaghan and A. Bazgir, Tetrahedron Lett., 2014, 55, 2366 CrossRef CAS; (e) D. Wang, D. Wang, L. Yan, G. Pan and J. Yang, Chin. Chem. Lett., 2016, 27, 953 CrossRef CAS; (f) J. M. Khurana, A. Chaudhary, B. Nand and A. Lumb, Tetrahedron Lett., 2012, 53, 3018 CrossRef CAS; (g) A. Rahmati and Z. Khalesi, Chin. Chem. Lett., 2012, 23, 1149 CrossRef CAS; (h) A. Z. M. S. Chowdhury and Y. Shibata, J. Heterocycl. Chem., 2001, 38, 743 CrossRef CAS; (i) R. B. Toche, B. K. Ghotekar, M. A. Kazi, D. B. Kendre and M. N. Jachak, Tetrahedron, 2007, 63, 8157 CrossRef CAS; (j) S. Abdolmohammadi and S. Karimpour, Chin. Chem. Lett., 2016, 27, 114 CrossRef CAS; (k) A. M. A. Al-Kadasi and G. M. Nazeruddin, J. Chem. Pharm. Res., 2013, 5, 204 CAS; (l) P. K. Ambre, R. R. S. Pissurlenkar, R. D. Wavhale, M. S. Shaikh, V. M. Khedkar, B. Wan, S. G. Franzblau and E. C. Coutinho, Med. Chem. Res., 2014, 23, 2564 CrossRef CAS.
- (a) S. Kobayashi, K. Manabe and S. Nagayama, in Modern Carbonyl Chemistry, ed. J. Otera, Wiley-VCH, Weinheim, 2000 Search PubMed; (b) K. Manabe, Y. Mori, T. Wakabayashi, S. Nagayama and S. Kobayashi, J. Am. Chem. Soc., 2000, 122, 7202 CrossRef CAS; (c) S. Kobayashi, S. Nagayama and T. Busujima, J. Am. Chem. Soc., 1998, 120, 8287 CrossRef CAS.
- (a) P. K. Sahu, P. K. Sahu, J. Lal, D. Thavaselvam and D. D. Agarwal, Med. Chem. Res., 2012, 21, 3826 CrossRef CAS; (b) P. K. Sahu, P. K. Sahu, Y. Sharma and D. D. Agarwal, J. Heterocycl. Chem., 2014, 51, 1193 CrossRef CAS; (c) P. K. Sahu, P. K. Sahu, S. K. Gupta, D. Thavaselvam and D. D. Agarwal, Eur. J. Med. Chem., 2012, 54, 366 CrossRef CAS PubMed; (d) P. K. Sahu, P. K. Sahu, D. Thavaselvam, A. M. Alafeefy and D. D. Agarwal, Med. Chem. Res., 2015, 24, 725 CrossRef CAS; (e) P. K. Sahu, P. K. Sahu, S. K. Gupta and D. D. Agarwal, Catal. Sci. Technol., 2013, 3, 1520 RSC; (f) P. K. Sahu, P. K. Sahu, S. K. Gupta and D. D. Agarwal, Ind. Eng. Chem. Res., 2014, 53, 2085 CrossRef CAS; (g) P. K. Sahu, P. K. Sahu, R. Jain, R. Yadav and D. D. Agarwal, Catal. Sci. Technol., 2012, 2, 2465 RSC.
- L. M. Wang, N. Jiao, J. Qui, J. J. Yu, J. Q. Liu, F. L. Guo and Y. Liu, Tetrahedron, 2010, 1, 339 CrossRef.
- H. Firouzabadi, N. Iranpoor and A. Garzan, Adv. Synth. Catal., 2005, 347, 1925 CrossRef CAS.
- Y. Watanabe, K. Sawada and M. Hayashi, Green Chem., 2010, 12, 384 RSC.
- D. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816 CrossRef CAS.
- (a) L. M. Wang, N. Jiao, J. Qiu, J. J. Yu, J. Q. Liu, F. L. Guo and Y. Liu, Tetrahedron, 2010, 1, 339 CrossRef; (b) Y. Watanabe, K. Sawada and M. Hayashi, Green Chem., 2010, 12, 384 RSC; (c) K. Manabe, Y. Mori and S. Kobayashi, Tetrahedron, 2001, 57, 2537 CrossRef CAS.
- Y. Moroi, N. Nishikido, H. Uehara and R. Matuura, J. Colloid Interface Sci., 1975, 50, 254 CrossRef CAS.
- A. Dey and G. N. Patwari, J. Chem. Sci., 2011, 123, 909 CrossRef CAS.
- L. Wang, N. Jiao, J. Qiu, J. Yu, J. Liu, F. Guo and Y. Liu, Tetrahedron, 2010, 66, 339 CrossRef CAS.
- A. Chatterjee, K. H. Sandip, M. Banerjee and P. K. Bhattacharya, Tetrahedron Lett., 2010, 51, 6700 CrossRef CAS.
- E. B. Mubofu and J. B. F. N. Engberts, J. Phys. Org. Chem., 2007, 20, 764 CrossRef CAS.
- J. F. Rathman, Curr. Opin. Colloid Interface Sci., 1996, 1, 514 CrossRef CAS.
- A. M. A. Al-Kadasi and G. M. Nazeruddin, J. Chem. Pharm. Res., 2013, 5, 204 CAS.
- M. Kidwai and P. Sapra, Synth. Commun., 2002, 32, 1639 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10801f |
| This journal is © The Royal Society of Chemistry 2016 |