DOI:
10.1039/C6RA10692G
(Paper)
RSC Adv., 2016,
6, 75430-75439
Cost effective biochar gels with super capabilities for heavy metal removal†
Received
25th April 2016
, Accepted 30th July 2016
First published on 1st August 2016
Abstract
A novel material, the biochar of a konjac-based material (KGMB), was prepared conveniently and economically with chemical activation of hydrothermally synthesized carbon nanospheres. Those carbon nanospheres have small sizes and high surface areas due to the high temperature sintering, which could be used for efficient adsorption of heavy metal ions such as Pb2+ and Cd2+. Detailed adsorption behaviors of the optimized biochar including adsorption isotherms and adsorption kinetics were investigated. The results indicated that the adsorption process is spontaneous, exothermic and pseudo-second-order chemisorption. When the temperature was 298 K, the KGMB dose was 5 mg, the initial concentration of Pb2+ and Cd2+ was both 50 mg L−1, the contact time was 24 h and pH of the ion solution was 5.5, the adsorption capacity reached up to 186.56 mg g−1 for Pb2+ and 129.67 mg g−1 for Cd2+, respectively. What's more, the adsorption capacities of Pb2+ and Cd2+ were 69.34 and 71.06 mg g−1, respectively, after adsorption–desorption process happened three times. Comparisons of the adsorption capacity of various adsorbents for Pb2+ and Cd2+, showed that this biochar is superior to many other adsorbents in terms of adsorption capacity and it is a cheap, efficient and accessible biochar. Thus, KGMB is a promising candidate for wastewater treatment.
1. Introduction
Heavy metal contamination is a constant serious problem at hazardous waste sites. Heavy metal contamination can occur in both soil and ground water, resulting from the rapid development of industrial activities such as mining, automobile manufacture, metal plating, and the nuclear industry. Uncontrolled development on landfill sites resulting from urbanization is another source of contamination of ground water. The contamination can be organic or inorganic. Among the various contaminations, heavy metal contamination is most dangerous because heavy metals are non-biodegradable and accumulative in living organisms. The main threats to human health from heavy metals are related to lead, cadmium, mercury, and arsenic. Lead and cadmium are not only serious concerns for ground water pollution but also constitute significant threats for humans.1–4 Although several adverse health effects of heavy metals have been known for a long time, heavy metal contamination continues, and is even increasing in some parts of the world. Heavy metal accumulation and storage have even been found in organisms' regeneration systems. In other words, it is very difficult to remove them from the body.
Several available technologies have been developed for heavy metal-contaminated waste stream and soil treatments. With the aim of minimizing the impacts of metal contamination, in situ solidification, ion-exchange, chemical precipitation, or membrane filtration have been utilized to remove non-biodegradable metallic compounds in ground water. However, the low efficiency and high cost of the processes of the most of these techniques has limited their applications. Sorbent-based techniques are widely used, although the cost of substrate materials and regeneration is a limiting factor. Therefore, the search for new alternatives to the widely used activated carbon is ongoing, from the perspective of finding a cheap, efficient, accessible adsorbent.
Biochar refers to carbonaceous residues from the incomplete burning of carbon-rich biomass under low temperature. Biochar can be fabricated from forestry and agricultural residues, industrial waste materials, animal manure, and the like.5–9 Biochars produced from bark or chestnut shell can significantly reduce the amount of Pb2+ and Cd2+ in ground water contamination. Biochars have also been produced from corn straw, hardwood, leather waste, or olive pomace, and similar properties were found.10–18 The hydrothermal method is a fast and effective accession to make biochar. Comparing with the other carbonaceous materials already mentioned, hydrothermal carbon possesses more oxygen-containing functional groups and active sites on the surface.19,20 To our knowledge, however, no reports exist about the use of biochar or hydrothermal carbon made from konjac glucomannan (KGM) for the removal of heavy metal contamination. KGMs that are extracted from amorphous konjac are composed of mannose and glucose in a molar ratio of 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a β-1,4-linkage, and contain acetyl group every 12 or 18 repeating units. Some branches of KGM are present at the C-3 position of KGM.21,22 As a renewable natural polymer, KGM exhibits excellent biocompatible and biodegradable properties.21 It also possesses the great advantages of gelling, film-forming, antibacterial action and low caloric value, facilitating its broad application in food, chemical, biological, and medical industries. Moreover, KGM is a promising adsorbent for the removal of heavy metal, due to the abundance of hydroxyl groups in its polymer chains. Liu et al. found that KGMs exhibited adsorption ability for both heavy metals and organic contaminations.23 Nevertheless, KGM cannot be used directly as an adsorbent because of its high swelling behavior in aqueous solution; thus, chemical modifications are necessary to change the swelling behavior of KGM.
1 with a β-1,4-linkage, and contain acetyl group every 12 or 18 repeating units. Some branches of KGM are present at the C-3 position of KGM.21,22 As a renewable natural polymer, KGM exhibits excellent biocompatible and biodegradable properties.21 It also possesses the great advantages of gelling, film-forming, antibacterial action and low caloric value, facilitating its broad application in food, chemical, biological, and medical industries. Moreover, KGM is a promising adsorbent for the removal of heavy metal, due to the abundance of hydroxyl groups in its polymer chains. Liu et al. found that KGMs exhibited adsorption ability for both heavy metals and organic contaminations.23 Nevertheless, KGM cannot be used directly as an adsorbent because of its high swelling behavior in aqueous solution; thus, chemical modifications are necessary to change the swelling behavior of KGM.
The present work focuses on the synthesis, characterization, and application of KGM biochar (KGMB) spheres on the removal of heavy metal ions (Pb2+ and Cd2+). KGMB spheres with a uniform spherical shape were chosen as the adsorbents in the adsorption experiments. Taking a panoramic view of the situation, KGMB can be an easily available, cheap, environmentally friendly, and efficient adsorbent.
2. Experiment
2.1 Materials
Konjac glucomannan (M = 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), sodium hydroxide and hydrochloric acid were purchased from Chengdu Kelong Chemical Reagent Factory Co., Ltd., China. The different concentration of Pb2+ and Cd2+ were gained by dissolving certain amount of Pb(NO3)2 and Cd(NO3)2·4H2O which were purchased from Kelong Chemicals, China in ultrapure water. Other reagents were purchased from Chengdu Forest Science & Technology Development Co., Ltd., China. All reagents used were of AR grade.
000), sodium hydroxide and hydrochloric acid were purchased from Chengdu Kelong Chemical Reagent Factory Co., Ltd., China. The different concentration of Pb2+ and Cd2+ were gained by dissolving certain amount of Pb(NO3)2 and Cd(NO3)2·4H2O which were purchased from Kelong Chemicals, China in ultrapure water. Other reagents were purchased from Chengdu Forest Science & Technology Development Co., Ltd., China. All reagents used were of AR grade.
2.2 Preparation of KGMB microspheres
The hydrothermal carbonization of KGM can be conducted as follows. At the very beginning, KGM powders are dissolved in deionized water forming a certain concentration solution. The solution is stirred at room temperature for 2 to 4 h and then the formed KGM solution is transferred into a Teflon-lined stainless steel autoclave (40 mL), sealed and heated in an oven keeping at 180 °C for 12 h. After cooling down to room temperature, KGM hydrothermal carbonized microspheres can be collected by centrifuge at a speed of 1500 rpm for 5 min (Thermo Electron LED D-37520 Osterode, Germany). The collected black powders are washed three times with deionized water until filtrate was nearly neutral and dried in a vacuum oven at 60 °C for 24 h.
2.3 Characterization of KGMB microspheres
Scanning electron microscopy (SEM) microphotographs and the elementary composition analysis were obtained with a Carl Zeiss Ultra 55 field emission SEM (FE-SEM) equipped with an energy-dispersive X-ray (EDX) detector operating at 15 kV. The specific surface area was measured by BET (JW-BK112, Beijing JWGB Sci. & Tech. Co., Ltd, China). The chemical functional groups of the KGMB were investigated by Fourier transform infrared (FTIR) spectra (Nicolet-5700, PerkinElmer, USA). The thermal stability of KGMB was investigated by a simultaneous thermal analyzer (Q500 TGA, TA Instruments, USA) in a range of temperature 20–800 °C at the heating rate of 10 °C min−1. The surface of adsorbent before and after Pb2+ and Cd2+ adsorption was measured by X-ray photoelectron spectroscopy (Escalab 250xi, ThermoScientific, USA). The zeta potentials of the KGMB at different pH values were determined by a Zetameter (ZetaPALS, USA). The average particle size of KGMB was measured by a laser size analyzer (90plus, Brookhaven Instruments, USA). Atomic adsorption spectroscopy (AAS, AA700, Perkin Elmer, USA) was used to explore the heavy metal ion concentrations in all batch adsorption experiments.
2.4 Batch adsorption ability
To study the Pb2+ and Cd2+ removal effect of KGMB, batch adsorption experiments were carefully performed. During the batch adsorption experiments, different initial pH values, heavy metal ion concentrations, temperatures, adsorbent concentrations, and contact times were systematically investigated. For all the adsorption experiments, the experimental conditions were strictly constrained as follows: 5 mg of KGMB powders were dispersed into 50 mL deionized water in a conical flask (100 mL) and all conical flasks were put into an orbital shaker, shaking at 150 rpm for 24 h. During the adsorption experiments, pH values were adjusted by 0.1 M HCl and 0.1 M NaOH solutions into the desired range of 2–6 and detected with pH meter (PHS-3BW, Shanghai bante Co., Ltd., China). The adsorbate concentration adopted 0.05 g L−1 in all adsorption operation except the study of the effect of the initial metal concentration. After the shaking, filtration was undertaken to separate adsorbent from adsorbate. The ion concentrations of the initial solution and filtrate were measured by AAS. For comparing the ion concentrations of the solution before and after the adsorption experiment, the adsorption amount at the equilibrium state (qe) was calculated by eqn (1):| |
 | (1) |
where qe (mg g−1) is the equilibrium adsorption amount of ion, c0 and ce (mg L−1) are the initial and equilibrium ion concentrations respectively, m (g) represents the weight of the dried adsorbent and V (L) is the volume of the ion solution. Mean values were used in all data analysis.
The regeneration and recyclability of the adsorbents were carried out according to Tang et al. procedure.24 50 mg of adsorbent loaded Pb2+ and Cd2+ were added separately into 500 mL, 0.1 mol L−1 HCl solution and the mixtures were shaken by an orbital shaker for 24 h with 150 rpm at 25 °C. Separated the supernatant by centrifugation and the solid was washed with deionized water for three times, 5 mg of stripped KGMB employed for another adsorption in triplicate. Three times for consecutive adsorption–desorption process were executed and the adsorption capacity of the adsorbent regenerated for three times was acquired.
3. Results and discussion
3.1 Structural properties
SEM microphotographs of the intrinsic KGM powder and KGMB spheres are shown in Fig. 1a and b. The morphology of the KGMBs with the different (weight/volume) ratios of KGM and solvent were shown in Fig. S1.† Among the four samples, KGMB with the ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 has the regular spherical appearance with the smallest radius of 200 nm and good uniformity. The mean diameters of the four KGMBs are listed in Table S1.† KGMB with KGM
8 has the regular spherical appearance with the smallest radius of 200 nm and good uniformity. The mean diameters of the four KGMBs are listed in Table S1.† KGMB with KGM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent = 1.0
solvent = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 was chosen to adsorbed Pb2+ and Cd2+. Compared with KGM powders, the specific surface area of nanospheres greatly increased, a feature that can significantly contribute to heavy metal removal. The specific surface area of intrinsic KGM and KGMB are 8 m2 g−1 and 17 m2 g−1 respectively. The EDX spectra of materials are shown in Fig. 1c–e and the atomic ratio of element are shown in Table 1, where it can be seen that the main element contents of KGMB are different before and after adsorption, demonstrating that Pb2+ and Cd2+ have been successfully adsorbed onto the KGMB. Fig. 1f shows the molecular structure of KGM.
8 was chosen to adsorbed Pb2+ and Cd2+. Compared with KGM powders, the specific surface area of nanospheres greatly increased, a feature that can significantly contribute to heavy metal removal. The specific surface area of intrinsic KGM and KGMB are 8 m2 g−1 and 17 m2 g−1 respectively. The EDX spectra of materials are shown in Fig. 1c–e and the atomic ratio of element are shown in Table 1, where it can be seen that the main element contents of KGMB are different before and after adsorption, demonstrating that Pb2+ and Cd2+ have been successfully adsorbed onto the KGMB. Fig. 1f shows the molecular structure of KGM.
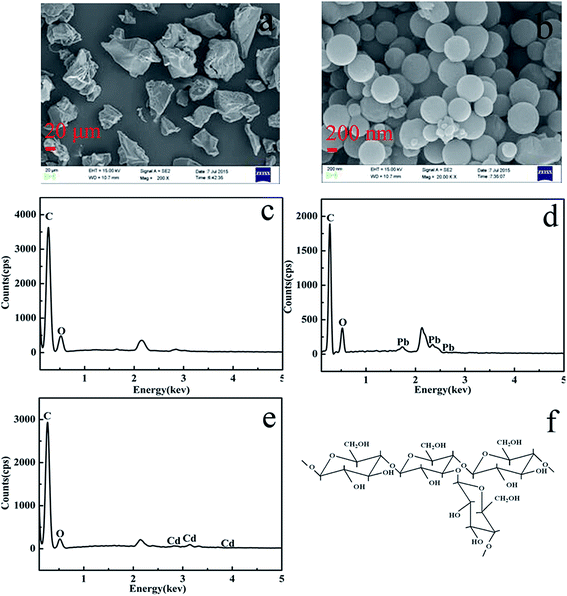 |
| | Fig. 1 SEM images of (a) KGM and (b) KGMB, EDX micrographs of KGMB (c) before and (d and e) after adsorption and (f) the structure of KGM. | |
Table 1 The atomic ratio of element from EDX
| Element |
Atomic% |
| KGMB |
KGMB-Pb |
KGMB-Cd |
| C |
79.35 |
84.84 |
75.49 |
| O |
20.65 |
14.75 |
24.12 |
| Pb |
0 |
0.39 |
0 |
| Cd |
0 |
0 |
0.41 |
FTIR is important in the study of the molecular structure of KGM. The intensity and width of the adsorption bands, as well as the position of the peaks, are all directly related to changes of conformation of macromolecules on the molecular level. The FTIR spectra, which could exert the differences and similarities between intrinsic KGM and KGMB before and after adsorption, were shown in Fig. 2. Our results were consistent with former reports.21–23 The characteristic absorption bands of the mannose in the KGM appeared at 808 and 881 cm−1. The adsorption band of 3435 cm−1 is the stretching peak of –OH groups. It is obvious that, after the hydrothermal treatment, many –OH groups are held, as demonstrated by the 3434 cm−1 peak of KGMB. For KGMB, the bands at 1630 cm−1 were intramolecular hydrogen bonds. The bands at 2924 cm−1 were assigned to the C–H stretching while the bands at 1398 cm−1 were attributed to methoxy group stretching vibrations.25 After adsorption of Pb2+ and Cd2+, the bands of all were shifted to long wavelength and the intensities of –OH at around 3400 cm−1 were weakened, implying that the KGMB has adsorbed Pb2+ and Cd2+ which was interconnected with –OH of KGMB.24
 |
| | Fig. 2 The FTIR spectra of KGM, KGMB before and after adsorption. | |
3.2 Thermal properties analysis
Thermogravimetric analysis (TGA) is a common method for exploring the thermal stability of materials. The thermal profiles of KGM and KGMB in the range of 0–800 °C are shown in Fig. 3. The whole profiles can be approximately divided into three parts seen in the curve of DTG-KGMB. Obviously, KGMB has much better thermal stability than that of KGM. For KGMB, in the initial stage, the weight loss of about 9.74% within the temperature range of 70–260 °C is mainly attributed to the evaporation of water physically and chemically adsorbed onto the KGMB. The second weight loss increases to 10.82% in the temperature range of 260–355 °C, indicating the initial degradation of KGMB. When the temperature exceeds 355 °C, the most significant weight loss occurs at the stage and the weight loss is 27.80%, which suggests the destruction and decomposition of the basic skeleton of KGMB. The TGA analysis indicates visibly that KGMB has a good thermostability.
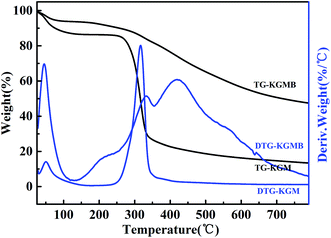 |
| | Fig. 3 Thermogravimetric analysis (TGA) of KGM and KGMB. | |
3.3 Surface analysis of KGMB
To confirm the ions were absorbed onto the surface of KGMB by chemical bonding, XPS analysis of KGMB before and after adsorption were performed. The results can be shown in Fig. 4, the new peaks at the binding energy of 143.8 eV and 139.9 eV appeared after Pb2+ adsorbed. The photoelectron peak of Pb2+ indicated the existence of Pb on the surface of KGMB. XPS surveys showed doublets characteristics of Pb appeared at 138.0 eV and 143.0 eV for Pb 4f7/2 and Pb 4f5/2 respectively after adsorption. The peak observed at the 138.0 eV reported for PbO.26
 |
| | Fig. 4 (a) XPS survey spectrum of adsorbents before and after adsorption, XPS spectra of O 1s for (b) KGMB, (c) KGMB carried Pb and (d) KGMB carried Cd. | |
The binding energy peaks were 412.0 eV and 404.2 eV for Cd 3d3/2 and Cd 3d5/2 after adsorption process, this indicated that Cd presented on the surface of KGMB. From Fig. 4b–d, it can be seen that before adsorption, O 1s prominent peaks located at 532.7 eV. After adsorption, the prominent peaks for O 1s were at the binding energy of 532.8 eV for Pb and 532.9 eV for Cd, peaks shift to a binding energy by about 0.1 eV and 0.2 eV, respectively. These illustrated that new material species which may be Pb–O and Cd–O were formed. The results indicated that the adsorption of Pb2+ and Cd2+ be mainly attributed to the replacement of the H+ existed –OH in KGMB.
3.4 Effect of initial pH values
The effect of pH values on the adsorption capacity of KGMB for Pb2+ and Cd2+ were measured in distinct solutions with pH values ranging from 2 to 6. The adsorption capacity of KGMB has not been investigated in the higher pH range (pH > 6) for the formation of lead hydroxide and cadmium hydroxide deposition.27 It can be seen in Fig. 5a, the adsorption amount for Pb2+ increases rapidly between 2 and 6 whereas for Cd2+, the curve exhibits a trend of first rising and then leveling off. KGMB didn't exhibits an apparent adsorption capacity for both Pb2+ and Cd2+ at pH = 2. This can be understood from the aspect of the hygroscopicity of KGMB. At a lower pH, there are lots of H+ ions in the solution which can easily form H3O+ with H2O. There will be a competition adsorption process between metal ions and the new forming H3O+ on KGMB. With the increasing of the pH values, the adsorption ability of KGMB for both Pb2+ and Cd2+ became better which can be intuitively found in Fig. 5a. This can be illustrated that the amount of H+ will obviously decrease with the increasing of pH values. Thus, the amount of H3O+ will decrease accordingly. The competition would become weaker between H3O+ and metal ions in turn. The surface charge of adsorbent detected by Zetamater in different pH values from 2 to 6 is shown in Fig. 5b, the isoelectric point of KGMB is achieved at pH = 2.3. The zeta potential of KGMB is positive at pH < 2.3 and is negative at pH > 2.3, which can explain why the adsorption capacity increased with the increasing of pH values. The zeta potential of KGMB tends to be stable (about −55 mV) in the pH range of 5 to 6. Thus, the optimal pH value of adsorption capacity is chosen to be around 5.5 in subsequent studies.
 |
| | Fig. 5 The effect of solution pH values on (a) adsorption and (b) zeta potential of KGMB. | |
3.5 Effect of adsorbent dose
The adsorption capacities for Pb2+ and Cd2+ were studied at the adsorbent dose range of 5 to 25 mg. All the results are shown in Fig. 6. The adsorption capacities of KGMB decreased with the increasing of adsorbent dose for both Pb2+ and Cd2+. The largest capacities appeared when the adsorbent dose is about 5 mg shown in Fig. 6b. So under a low dose of adsorbent, the adsorbent presented good adsorption properties towards Pb2+ and Cd2+ due to there existed –OH groups and active site exposed completely of adsorbent. However, the adsorption capacity weakened gradually with the increasing of adsorbent dose, it's may be attributed to adsorption active sites remaining unsaturated when the adsorption system reached equilibrium. The adsorbed metal concentrations were increased very slowly with the increasing amount of adsorbent in error range as shown in Fig. 6a, which indicated that the adsorption behavior of adsorbent dose was reasonable. Therefore, 5 mg of adsorbent was selected and used in the subsequent batch experiments.
 |
| | Fig. 6 (a) The adsorbed metal concentrations of different amount of adsorbent and (b) the effect of amount of adsorbent on Pb2+ and Cd2+ adsorption capacity of KGMB. | |
3.6 Adsorption isotherm study
In order to understand the adsorption behavior of KGMB for Pb2+ and Cd2+, the effect of the initial concentration of adsorbate was studied with the concentration range from 0.010 to 0.125 g L−1. The curves reflecting adsorption capacity towards ions are displayed in Fig. 7. The adsorption curves of Pb2+ and Cd2+ reached their highest values at about 0.073 g L−1 and 0.062 g L−1, respectively, in the operable concentration range. But considering actual situation, the whole adsorption processes were carried out at 0.050 g L−1.
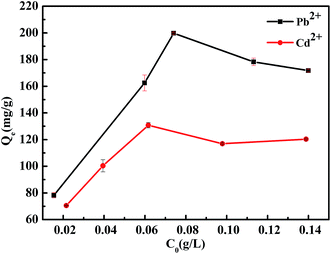 |
| | Fig. 7 Effect of initial concentration on adsorption capacity of KGMB for Pb2+ and Cd2+. | |
The Langmuir and Freundlich models are considered to identify the mechanism of the adsorption process. The model parameters can be calculated according to eqn (2). The fitting curve of the Langmuir model is shown in Fig. 8 and the values are listed in Table 2.
| |
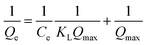 | (2) |
where
Qe (mg g
−1) is the equilibrium adsorption amount,
Qmax (mg g
−1) is the maximum ion adsorption capacity of the monolayer, and
Ce is the equilibrium concentration.
KL is a Langmuir constant representing energy of adsorption. The important properties of the Langmuir adsorption isotherm can be presented by dimensionless equilibrium constant
RL, which is defined by
eqn (3).
| |
 | (3) |
where
C0 (mg L
−1) is initial concentration of ions. The value of
RL will be in the range of 0–1, indicating the favorable adsorption of Pb
2+ and Cd
2+ onto the KGMB.
RL = 0 indicates the irreversible adsorption;
RL = 1, the adsorption processes are the linear adsorption;
RL > 1, Pb
2+ and Cd
2+ are difficult to be adsorbed. The Freundlich model describes multilayer adsorption of heterogeneous surfaces. That model is shown in the following
eqn (4).
| |
 | (4) |
where
KF and
n are the Freundlich constants,
KF (mg g
−1) represents multilayer adsorption capacity but an empirical parameter of
n stands for adsorption intensity, which can further indicate a favorable adsorption of ions at the value of 10 >
n > 1. It can be considered as surface adsorption.
 |
| | Fig. 8 Langmuir model for the adsorption of Pb2+ and Cd2+ onto KGMB. | |
Table 2 Characteristic parameters obtained from Langmuir adsorption isotherm model
| Initial ion concentration |
RL |
Initial ion concentration |
RL |
| Pb2+ |
15 |
0.44 |
Cd2+ |
22 |
0.42 |
| 59 |
0.17 |
39 |
0.29 |
| 74 |
0.14 |
62 |
0.20 |
| 113 |
0.09 |
98 |
0.13 |
| 139 |
0.08 |
139 |
0.09 |
| R2 |
0.984 |
R2 |
0.990 |
| Qmax (mg g−1) |
202.84 |
Qmax (mg g−1) |
137.55 |
| KL (L mg−1) |
0.083 |
KL (L mg−1) |
0.065 |
According to eqn (4), values of KF and n are found to be 44.33 and 3.05 for Pb2+ while 42.24 and 4.22 for Cd2+ calculated from the slope and the linear plot of Qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce. The values of the correlation coefficient R2 of Pb2+ and Cd2+ linear fittings are 0.744 and 0.514, respectively.
Ce. The values of the correlation coefficient R2 of Pb2+ and Cd2+ linear fittings are 0.744 and 0.514, respectively.
In comparison of the two isotherm models, the adsorption processes fit better to the Langmuir isotherm model according to the values of R2, regardless of the adsorption behavior for Pb2+ or Cd2+. This result further illustrates that the adsorption behaviors are mainly dependent on the Langmuir isotherm hypothesis.
3.7 Effect of contact time and kinetic studies
Adsorption experiments covering different contact times from 0 to 24 h were performed. The effects of contact time on the adsorption of ions to KGMB are shown in Fig. 9. The adsorption amount of Pb2+ increased sharply during the contact time of 220 min and it increased rapidly at 180 min for Cd2+. Adsorption equilibrium occurred at 490 min for Cd2+ and 600 min for Pb2+.
 |
| | Fig. 9 Effect of contact time on Pb2+ and Cd2+ adsorption capacity onto KGMB. | |
Investigation on identifying the controlling mechanism of adsorption was undertaken by three different kinetic models, namely pseudo-first-order, pseudo-second-order, and intra-particle diffusion, given in eqn (5)–(7) respectively.
| |
 | (5) |
| |
 | (6) |
where
t (min) refers to contact time,
k1 (min
−1) and
k2 (g mg
−1 min
−1) are the rate constants,
kp (mg g
−1 min
−1/2) is the intra-particle diffusion rate constant.
c (mg g
−1) is the constant proportional to the extent of boundary layer thickness.
qt and
qe are the adsorption capacity at any time
t and the adsorption amount under equilibrium, respectively.
The relative constants in eqn (5)–(7) are listed in Table 3 and the fitting multi-linearity plots of the intra-particle diffusion model are presented in Fig. S2.† From Fig. S2,† the qt verses t0.5 plot can be divided into three distinct regions, which indicated that the adsorption process could be considered as three individual stages. The first stage represents external diffusion which is distributed onto the outer surface of the biosorbent, the second stage describes the gradual biosorption which is known as the interior surface adsorption via active sites distributed to pores and the third stage was carried out and reached the adsorption equilibrium when the intra-particle diffusion started to slow down. Thus, the surface adsorption and intra-particle diffusion may simultaneously contribute to the rate-determining step during the process of Pb2+ and Cd2+ uptake by KGMB.
Table 3 Kinetic parameters for Pb2+ and Cd2+ adsorption onto KGMB
| qe,exp (mg g−1) |
Pseudo-first-order kinetics |
Pseudo-second-order kinetics |
Intraparticle diffusion |
| qe,cal (mg g−1) |
k1 (min−1) |
R2 |
qe,cal (mg g−1) |
k2 (g mg−1 min−1) |
R2 |
kp (mg g−1 min−1/2) |
R2 |
| Pb2+ |
186.56 |
106.99 |
0.00606 |
0.9573 |
176.06 |
0.000133 |
0.9956 |
4.7344 |
0.7633 |
| Cd2+ |
129.67 |
75.42 |
0.00313 |
0.9206 |
125.63 |
0.0000899 |
0.9900 |
1.8271 |
0.9998 |
Compared to the pseudo-first-kinetic model, the efficient value is highest where qe,cal is closer to qe,exp and the R2 values are highest in the pseudo-second-order kinetic model. This result indicates that the adsorption progress can be well described by the pseudo-second-order kinetic model, thus the adsorption of Pb2+ and Cd2+ on KGMB is chemisorptions process. For further interpretation, chemisorption determines that the adsorption operation is a rate-controlling step, it may be indicated that there are exist the covalent forces via electron sharing, electron transfer or electron exchange.28
3.8 Thermodynamic study
The effect of temperature on the adsorption of ions onto the adsorbent can be found in Fig. 10. From Fig. 10a, the adsorption capacity for Pb2+ decreases slightly from 25 °C to 65 °C, with the greatest amount being approximately 202.84 mg g−1 at 25 °C, whereas the maximum adsorption capacity of Cd2+ is about 137.55 mg g−1. Given the slight effect of temperature, 25 °C was adopted for all the batch adsorption experiments to save energy.
 |
| | Fig. 10 (a) Effect of temperature on adsorption capacity of KGMB for Pb2+ and Cd2+ and (b) the relationship between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K and 1/T for ion adsorption onto KGMB. K and 1/T for ion adsorption onto KGMB. | |
Thermodynamic parameters (ΔH0, ΔS0, ΔG0) were calculated by eqn (8)–(10) and are listed in Table 4. The fitting linear curves are shown in Fig. 10b.
| |
 | (8) |
| |
 | (9) |
| |
 | (10) |
where
kD is the distribution coefficient (L g
−1), and its value is related to the concentration and adsorption capacities of ions under the adsorption equilibrium condition. Gibbs free energy change (Δ
G0) is determined by the temperature and distribution coefficient of ions.
R is the universal gas constant (8.314 J mol
−1 K
−1).
T (K) is the absolute temperature. Enthalpy change (Δ
H0) and entropy change (Δ
S0) can be obtained
via the fitting linear curve (
Fig. 10b) of
eqn (8).
Table 4 Thermodynamic parameters for adsorption of Pb2+ and Cd2+ onto KGMB
| Ion species |
ΔH0 (kJ mol−1) |
ΔS0 (J mol−1 K−1) |
ΔG0 (kJ mol−1) |
| 298.15 K |
308.15 K |
318.15 K |
328.15 K |
338.15 K |
| Pb2+ |
−2.828 |
8.345 |
−5.185 |
−5.350 |
−5.422 |
−5.506 |
−5.522 |
| Cd2+ |
−6.957 |
−15.145 |
−2.313 |
−2.353 |
−2.137 |
−1.973 |
−1.748 |
From Table 4, the negative values of ΔH0 for adsorption of Pb2+ and Cd2+ imply that the adsorption processes are exothermic. With increasing temperature, the negative values of ΔG0 reduce slightly for Pb2+ but rise sharply for Cd2+, especially from 318.15 K to 338.15 K. These results reflect that the two adsorption processes are spontaneous under testing conditions and further indicate that the adsorption capacities of Pb2+ do not change obviously, whereas they change considerably for Cd2+ at different temperatures. The positive value of ΔS0 of Pb2+ indicates the good affinity of the adsorbent towards Pb2+ and the structural change indicates the increased randomness, yet the negative value of ΔS0 of Cd2+ indicates the decreased randomness at the solid–solution interface during the adsorption process.
3.9 Comparisons with other adsorbents
Comparisons of the adsorption capacity of various adsorbents for Pb2+ and Cd2+ are shown in Table 5. The results show that KGMB in this study was superior to many other adsorbents in terms of adsorption capacity and experiment conditions. Song et al.33 has used HNO3 and H2O2 to oxidize a commercially available activated carbon, and the modified activated carbon which exhibited better adsorption behavior of Pb2+, reached up to the maximum capacity of 40 mg g−1. It was also reported that animal manure biochar had an excellent adsorption capacity.31,32 Bagasse-based activated carbon could be used as a adsorbent for Cd2+, and some other kinds of biochar have also been prepared for Cd2+ adsorbents, such as water hyacinth biochar, switchgrass biochar and spent bleaching earth carbon.24,35–37 Our study indicated that KGMB could be a very useful adsorbent for Pb2+ and Cd2+, as the maximum adsorption capacity could be 202.84 mg g−1 for Pb2+ and 137.6 mg g−1 for Cd2+, respectively, compared to most adsorbents listed in Table 5 and thus KGMB can be used as an efficient and low cost sorbent for Pb2+ and Cd2+. Possible reasons might be the negative charges on the surface of KGMB microspheres, the small size of KGMB particles, and many –OH groups on the surface.
Table 5 Comparisons of adsorption capacities of biochar materials for Pb2+ and Cd2+
| Ions |
Adsorbent |
Dosage (g L−1) |
pH |
Temperature (K) |
Qmax (mg g−1) |
Reference |
| Pb2+ |
Alginate capsule biochar |
1.0 |
3.0–5.5 |
300 |
263.2 |
29 |
| Sludge-derived biochar |
4.0 |
2.0–5.0 |
298 |
30.9 |
30 |
| Pig manure biochar |
5.0 |
1.0–12.0 |
293 ± 1 |
175.4 |
31 |
| Dairy manure biochar |
5.0 |
Not controlled |
298 |
109.4 |
32 |
| Active carbon |
2.0 |
3.2–3.6 |
298 |
40.1 |
33 |
| KGMB |
0.1 |
2.0–6.0 |
298–338 |
202.8 |
Current study |
| Cd2+ |
APAM-MFX |
10–400 |
3.0–7.0 |
295–315 |
95.2 |
34 |
| Spent bleaching earth carbon |
1.0 |
1.0–6.0 |
303 |
46.7 |
35 |
| Water hyacinth BC450 |
5.0 |
7.2–10.4 |
299 |
70.3 |
24 |
| Switchgrass biochar |
2.0 |
5.0 |
296 ± 1 |
34.0 |
36 |
| Active carbon |
6.0 |
4.5 |
298 |
38.0 |
37 |
| KGMB |
0.1 |
2.0–6.0 |
298–338 |
137.6 |
Current study |
3.10 Desorption and reusability
Reusability studies are essential for determining the regeneration and effectiveness of the adsorbent. In this study, desorption was operated with batch experiments in 0.1 mol L−1 HCl aqueous solutions, since HCl is an important desorbing agent for recovering the adsorbent used for heavy metals removal,38,39 to investigate the regeneration ability of KGMB. The adsorption–desorption performance with the three-time consecutive process was evaluated and shown in Fig. 11. The adsorption capacities for Pb2+ and Cd2+ were observed to decrease evidently with the increasing cycles of adsorption, which might be attributed to their different hydration energy.40 After the three-time recycle process, the adsorption capacities of Pb2+ and Cd2+ were 69.34 and 71.06 mg g−1, respectively, which indicated that KGMB is an promising adsorbent.
 |
| | Fig. 11 Reusability of the KGMB for Pb2+ and Cd2+ adsorption. | |
4. Conclusions
A novel KGM-based adsorbent was produced by a one-pot hydrothermal method. The synthesis process was simple and environmentally friendly. More important, the adsorption of KGMB has seldom been studied by other groups; however, KGMB exhibits a very efficient adsorption effect for Pb2+ and Cd2+ in this study. The successful adsorption of Pb2+ and Cd2+ was demonstrated by EDX and XPS results. According to our systematic batch experiments, the maximum capturing capacity of KGMB reached 202.84 mg g−1 for Pb2+ and 137.55 mg g−1 for Cd2+ at pH 5.5, KGMB displays an excellent adsorption performance and reusable ability and after adsorption–desorption processes for three times, the adsorption capacities of Pb2+ and Cd2+ were 69.34 and 71.06 mg g−1, respectively. The adsorption processes are spontaneously thermodynamically favorable and exothermic in the temperature range from 298.15 K to 338.15 K. For Pb2+, the randomness of adsorption testing increases whereas for Cd2+, it decreased at the solid–solution interface. The adsorption processes follow Langmuir's isotherm model, demonstrating monolayer surface adsorption. The kinetic model belongs to a pseudo-second order kinetic chemisorption. So, KGMB is a promising material for heavy metal ions in water treatment.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (NSFC; Grant No. 31300793, 31400809) and Engineering Research Center of Biomass Materials (SWUST), Ministry of Education (No. 13zxbk03).
References
- Z. Li, Z. Ma, T. J. vanderKuijp, Z. Yuan and L. Huang, Sci. Total Environ., 2014, 468, 843–853 CrossRef PubMed.
- P. O. Boamah, Y. Huang, M. Hua, Q. Zhang, Y. Liu, J. Onumah, W. Wang and Y. Song, Carbohydr. Polym., 2015, 122, 255–264 CrossRef CAS PubMed.
- A. Ozer and H. B. Pirincci, J. Hazard. Mater., 2006, 137, 849–855 CrossRef CAS PubMed.
- J. Feber and M. Ahmed, Clin. Sci., 2010, 119, 151–161 CrossRef PubMed.
- J. J. Manya, Environ. Sci. Technol., 2012, 46, 7939–7954 CrossRef CAS PubMed.
- N. G. Kandile and A. S. Nasr, Carbohydr. Polym., 2009, 78, 753–759 CrossRef CAS.
- K. Kim, J. Kim, T. Cho and J. Choi, Bioresour. Technol., 2012, 118, 158–162 CrossRef CAS PubMed.
- M. K. Mondal, J. Environ. Manage., 2009, 90, 3266–3271 CrossRef CAS PubMed.
- G. Chen, X. Shan, Y. Wang, Z. Pei, X. Shen, B. Wen and G. Owens, Environ. Sci. Technol., 2008, 42, 8297–8302 CrossRef CAS PubMed.
- Y. Yang, Z. Wei, X. Zhang, X. Chen, D. Yue, Q. Yin, L. Xiao and L. Yang, Bioresour. Technol., 2014, 171, 227–232 CrossRef CAS PubMed.
- E. Forgacs, T. Cserhati and G. Oros, Environment International, 2004, 30, 953–971 CrossRef CAS PubMed.
- M. Han and Y. Yun, Biochem. Eng. J., 2007, 36, 2–7 CrossRef CAS.
- B. Royer, N. F. Cardoso, E. C. Lima, J. C. P. Vaghetti, N. M. Simon, T. Calvete and R. C. Veses, J. Hazard. Mater., 2009, 164, 1213–1222 CrossRef CAS PubMed.
- P. A. Carneiro, G. A. Umbuzeiro, D. P. Oliveira and M. V. B. Zanoni, J. Hazard. Mater., 2010, 174, 694–699 CrossRef CAS PubMed.
- F. I. Hai, K. Yamamoto and K. Fukushi, Environ. Sci. Technol., 2007, 37, 315–377 CrossRef CAS.
- B. Royer, N. F. Cardoso, E. C. Lima, V. S. O. Ruiz, T. R. Macedo and C. Airoldi, J. Colloid Interface Sci., 2009, 336, 398–405 CrossRef CAS PubMed.
- E. Demirbas, M. Kobya and M. T. Sulak, Bioresour. Technol., 2008, 99, 5368–5373 CrossRef CAS PubMed.
- F. M. Machado, C. P. Bergmann, E. C. Lima, B. Royer, F. E. de Souza, I. M. Jauris, T. Calvete and S. B. Fagan, Phys. Chem. Chem. Phys., 2012, 14, 11139–11153 RSC.
- M. Sevilla and A. B. Fuertes, Chem.–Eur. J., 2009, 15, 4195–4203 CrossRef CAS PubMed.
- H. Wang, L. Ma, K. Cao, J. Geng, J. Liu, Q. Song, X. Yang and S. Li, J. Hazard. Mater., 2012, 229, 321–330 CrossRef PubMed.
- I. Ratcliffe, P. A. Williams, C. Viebke and J. Meadows, Biomacromolecules, 2005, 6, 1977–1986 CrossRef CAS PubMed.
- M. A. Williams, T. J. Foster, D. R. Martin, I. T. Norton, M. Yoshimura and K. Nishinari, Biomacromolecules, 2000, 1, 440–450 CrossRef CAS PubMed.
- F. Liu, X. Luo, X. Lin, L. Liang and Y. Chen, J. Hazard. Mater., 2009, 171, 802–808 CrossRef CAS PubMed.
- J. Tang, B. Mu, M. Zheng and A. Wang, ACS Sustainable Chem. Eng., 2015, 3, 1125–1135 CrossRef CAS.
- D. Tian, X. Wu, C. Liu and H. Xie, J. Appl. Polym. Sci., 2010, 115, 2368–2374 CrossRef CAS.
- S. M. Maliyekkal, K. P. Lisha and T. Pradeep, J. Hazard. Mater., 2010, 181, 986–995 CrossRef CAS PubMed.
- P. Wang, M. Du, H. Zhu, S. Bao, T. Yang and M. Zou, J. Hazard. Mater., 2015, 286, 533–544 CrossRef CAS PubMed.
- M. R. Awual, M. A. Shenashen, H. Yaita and A. Jyo, Water Res., 2012, 46, 5541–5550 CrossRef CAS PubMed.
- X. Do and B. Lee, J. Environ. Manage., 2013, 131, 375–382 CrossRef CAS PubMed.
- H. Lu, W. Zhang, Y. Yang, X. Huang, S. Wang and R. Qiu, Water Res., 2012, 46, 854–862 CrossRef CAS PubMed.
- D. Kolodynsk, R. Wnetrzak, J. J. Leahy, M. H. B. Hayes, W. Kwapinski and Z. Hubicki, Chem. Eng. J., 2012, 197, 295–305 CrossRef.
- X. Cao, L. Ma, B. Gao and W. Harris, Environ. Sci. Technol., 2009, 43, 3285–3291 CrossRef CAS PubMed.
- X. Song, H. Liu, L. Cheng and Y. Qu, Desalination, 2010, 255, 78–83 CrossRef CAS.
- W. Wei, A. Li, J. Yang, F. Ma, D. Wu, J. Xing, X. Zhou and D. Zhao, Bioresour. Technol., 2015, 175, 34–41 CrossRef CAS PubMed.
- F. Zhang, X. Wang, D. Yin, B. Peng, C. Tan, Y. Liu, X. Tan and X. Wu, J. Environ. Manage., 2015, 153, 68–73 CrossRef CAS PubMed.
- P. Regmi, J. L. G. Moscoso, S. Kumar, X. Cao, J. Mao and G. Schafran, J. Environ. Manage., 2012, 109, 61–69 CrossRef CAS PubMed.
- D. Mohan and K. P. Singh, Water Res., 2002, 36, 2304–2318 CrossRef CAS PubMed.
- Y. Zhou, H. Nie, C. B. White, Z. He and L. Zhu, J. Colloid Interface Sci., 2009, 330, 29–37 CrossRef CAS PubMed.
- X. Wang, Y. Zheng and A. Wang, J. Hazard. Mater., 2009, 168, 970–977 CrossRef CAS PubMed.
- S. Wan, Z. Ma, Y. Xue, M. Ma, S. Xu, L. Qian and Q. Zhang, Ind. Eng. Chem. Res., 2014, 53, 3629–3635 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10692g |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *d
*d
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a β-1,4-linkage, and contain acetyl group every 12 or 18 repeating units. Some branches of KGM are present at the C-3 position of KGM.21,22 As a renewable natural polymer, KGM exhibits excellent biocompatible and biodegradable properties.21 It also possesses the great advantages of gelling, film-forming, antibacterial action and low caloric value, facilitating its broad application in food, chemical, biological, and medical industries. Moreover, KGM is a promising adsorbent for the removal of heavy metal, due to the abundance of hydroxyl groups in its polymer chains. Liu et al. found that KGMs exhibited adsorption ability for both heavy metals and organic contaminations.23 Nevertheless, KGM cannot be used directly as an adsorbent because of its high swelling behavior in aqueous solution; thus, chemical modifications are necessary to change the swelling behavior of KGM.
1 with a β-1,4-linkage, and contain acetyl group every 12 or 18 repeating units. Some branches of KGM are present at the C-3 position of KGM.21,22 As a renewable natural polymer, KGM exhibits excellent biocompatible and biodegradable properties.21 It also possesses the great advantages of gelling, film-forming, antibacterial action and low caloric value, facilitating its broad application in food, chemical, biological, and medical industries. Moreover, KGM is a promising adsorbent for the removal of heavy metal, due to the abundance of hydroxyl groups in its polymer chains. Liu et al. found that KGMs exhibited adsorption ability for both heavy metals and organic contaminations.23 Nevertheless, KGM cannot be used directly as an adsorbent because of its high swelling behavior in aqueous solution; thus, chemical modifications are necessary to change the swelling behavior of KGM.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), sodium hydroxide and hydrochloric acid were purchased from Chengdu Kelong Chemical Reagent Factory Co., Ltd., China. The different concentration of Pb2+ and Cd2+ were gained by dissolving certain amount of Pb(NO3)2 and Cd(NO3)2·4H2O which were purchased from Kelong Chemicals, China in ultrapure water. Other reagents were purchased from Chengdu Forest Science & Technology Development Co., Ltd., China. All reagents used were of AR grade.
000), sodium hydroxide and hydrochloric acid were purchased from Chengdu Kelong Chemical Reagent Factory Co., Ltd., China. The different concentration of Pb2+ and Cd2+ were gained by dissolving certain amount of Pb(NO3)2 and Cd(NO3)2·4H2O which were purchased from Kelong Chemicals, China in ultrapure water. Other reagents were purchased from Chengdu Forest Science & Technology Development Co., Ltd., China. All reagents used were of AR grade.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 has the regular spherical appearance with the smallest radius of 200 nm and good uniformity. The mean diameters of the four KGMBs are listed in Table S1.† KGMB with KGM
8 has the regular spherical appearance with the smallest radius of 200 nm and good uniformity. The mean diameters of the four KGMBs are listed in Table S1.† KGMB with KGM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent = 1.0
solvent = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 was chosen to adsorbed Pb2+ and Cd2+. Compared with KGM powders, the specific surface area of nanospheres greatly increased, a feature that can significantly contribute to heavy metal removal. The specific surface area of intrinsic KGM and KGMB are 8 m2 g−1 and 17 m2 g−1 respectively. The EDX spectra of materials are shown in Fig. 1c–e and the atomic ratio of element are shown in Table 1, where it can be seen that the main element contents of KGMB are different before and after adsorption, demonstrating that Pb2+ and Cd2+ have been successfully adsorbed onto the KGMB. Fig. 1f shows the molecular structure of KGM.
8 was chosen to adsorbed Pb2+ and Cd2+. Compared with KGM powders, the specific surface area of nanospheres greatly increased, a feature that can significantly contribute to heavy metal removal. The specific surface area of intrinsic KGM and KGMB are 8 m2 g−1 and 17 m2 g−1 respectively. The EDX spectra of materials are shown in Fig. 1c–e and the atomic ratio of element are shown in Table 1, where it can be seen that the main element contents of KGMB are different before and after adsorption, demonstrating that Pb2+ and Cd2+ have been successfully adsorbed onto the KGMB. Fig. 1f shows the molecular structure of KGM.



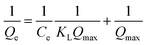


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce. The values of the correlation coefficient R2 of Pb2+ and Cd2+ linear fittings are 0.744 and 0.514, respectively.
Ce. The values of the correlation coefficient R2 of Pb2+ and Cd2+ linear fittings are 0.744 and 0.514, respectively.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K and 1/T for ion adsorption onto KGMB.
K and 1/T for ion adsorption onto KGMB.









