Anatase TiO2 nanosheets with exposed highly reactive (001) facets as an efficient photoanode for quantum dot-sensitized solar cells†
Haitao Zhoua,
Lin Li*a,
Dianli Jianga,
Yingbing Lua and
Kai Panb
aKey Laboratory for Photonic and Electronic Bandgap Materials, Ministry of Education, School of Physics and Electronic Engineering, Harbin Normal University, Harbin 150025, P. R. China. E-mail: physics_lin@hotmail.com
bKey Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, Heilongjiang University, Harbin 150080, P. R. China
First published on 8th July 2016
Abstract
Two-dimensional (2D) anatase TiO2 nanosheets (TiO2-NSs) with exposed (001) crystal planes were obtained via a simple one-pot hydrothermal route. And they were utilized to fabricate efficient CdSe quantum dot sensitized solar cells (QDSSCs) for the first time. A power conversion efficiency (PCE) of 5.01% was attained with the TiO2-NS based cell, which is 63% higher than that of the TiO2 nanoparticle (TiO2-NP) based cell under the same conditions. The increased photovoltaic performance mainly profits from more quantum-dot (QD) loading onto TiO2-NSs due to large pore size and the strong absorption ability with the carboxylate linker of colloidal quantum dots capped using bifunctional linker molecules in the TiO2-NSs with exposed (001) crystal planes, which lead to an enhancement of light harvesting efficiency and thus significantly enhanced short-circuit photocurrent. Furthermore, the good crystallization, large particle size and low surface area of the TiO2-NSs also result in fewer defects and provide efficient electron transfer pathways and prolong electron lifetime. Meanwhile, the large pore size of the TiO2-NS photoanode will also diminish the infiltration resistance of the electrolyte, which is helpful to regenerate the quantum-dots (QDs). The excellent properties of the 2D anatase TiO2 nanosheet (TiO2-NS) with exposed (001) crystal planes make it a promising candidate as a photoanode material for highly efficient QDSSCs.
1. Introduction
The development of high-performance and low-cost solar devices for clean and sustainable energy have drawn intense attention in both the scientific and industrial domain.1,2 Among the photovoltaic devices, dye-sensitized solar cells (DSSCs), with the advantages of environmental benignity and low cost, have been regarded as one of the most promising alternatives for efficient solar energy conversion.3–6 However, the sensitizer of DSSCs commonly uses organic dyes of ruthenium polypyridine complexes. To improve the stability of the sensitizer, decrease the loss and enhance light harvesting in the visible light region, much effort has been made to develop high performance sensitizers. For this reason, narrow-band-gap QDs have been widely investigated due to their excellent optical and electronic properties, namely, tunable band gap depending on the QD size, high extinction coefficient and generation of multiple exitons,1,7,8 which could lead to 44% theoretical photovoltaic conversion in QDSSCs. Such an efficiency is much higher than the 31% for semiconductor solar cells according to the Schockley–Queisser limit.9Although the efficiency of QDSSCs has experienced fast growth in the last year, the reported highest efficiency is still less than 12%,10,11 which lags remarkably behind the theoretical photovoltaic conversion of QDSSCs. Until now, many research studies have been conducted to improve the PCE of QDSSCs, including constructing a novel photoanode with suitable porosity and absorption ability for quantum dots,12 designing new semiconductor QDs with a broad wavelength range of optical absorption,13 synthesis of suitable electrolytes that scavenge photogenerated holes effectively and a more positive redox potential to gain high open voltage,14 and selecting counter electrodes with high conductivity and electrocatalytic activity.15 Although the QDSSCs can be regarded as a derivative of DSSCs, the inorganic quantum dots are significantly different from the organic dye. In particularly, the inorganic colloidal quantum dots are much bigger (the size of the colloidal QDs capped with bifunctional linker is ∼5 nm) than the dye molecules (∼0.5 nm). So, the optimized photoanode in DSSCs is not suitable for QDSSCs. In order to exploit the potential of QDSSCs and obtain higher PCEs, it is very essential to develop an optimized photoanode with suitable porosity for the filling of colloidal QDs and strong absorption ability for the colloidal QDs. Based on the above reasons, considerable efforts have been focused on improving the performance of QDSSCs through the design and optimization of TiO2 mesoporous films to increase the QDs loading, inhibit charge recombination, and shorten charge transmission path.1,12,16,17 In general, TiO2 mesoporous nanoparticle films are the most frequently used photoanodes in DSSCs or QDSSCs due to their high internal surface area that maximizes the adsorption of sensitizers.18–20 However, a major drawback of the mesoporous nanoparticle photoanode is the inefficient electron transport due to defects such as grain boundaries and surface traps.8,21,22 The rapid transport of photogenerated electrons in the porous TiO2 films is pivotal to improve the photoelectric conversion efficiency of cells and reduce the recombination rate of photo-generated electron–hole pairs. One solution to the drawbacks of TiO2-NPs is to use one-dimensional (1D) nanostructures, which can provide direct channels for the rapid collection of the photogenerated electrons, reduction of charge recombination, and simultaneously enhance the electrolyte diffusion.23–25 However, the typical low surface area of 1D nanostructures leads to reduced QD loading, and hence reduced light harvesting, resulting in lower photocurrent.21,22 Alternatively, to increase the internal surface area and light-harvesting efficiency, three-dimensional (3D) nanostructures or hybrid nanostructures have been introduced in QDSSCs.21,22,26,27 This enhancement in efficiency is mainly due to the fact that more QDs are adsorbed on the photoanode. Most of these structures, however, were obtained based on the one or two materials by multi-step hydrothermal process, which unavoidably bring tedious fabrication procedures and lots of surface trap states.
Among all kinds of morphology, 2D TiO2-NSs with exposed (001) crystal planes have attracted more attention due to their “high-energy” crystal planes that are favorable for more sensitizers adsorption and charge separation, which exhibited an outstanding performance in DSSCs.5,28–33 Such as, Yu et al. prepared DSSCs based on anatase TiO2 nanosheets with exposed (001) crystal planes, which showed enhanced power conversion efficiency compared with TiO2 nanoparticles and Degussa P25 solar cells.28 The enhanced performance is attributed to the good crystallization, high pore volume, large particle size and enhanced light scattering of TiO2 nanosheets. Wang and co-workers reported the fabrication of DSSCs based on a TiO2 nanosheet/nanoparticle gradient photoanode film, which also showed an enhanced photovoltaic performance due to the relatively high surface area of TiO2 nanosheet and enhanced light scattering capability of gradient film as well as efficient electron transport from dye molecules to the conduction band of TiO2.29 In the QDSSCs, You et al. fabricated QDSSCs by in situ sensitizing CdS QDs on the TiO2 nanosheets photoanode with exposed (001) and (100) crystal planes using successive ionic layer adsorption and reaction (SILAR) method.31 The photovoltaic conversion efficiencies were 2.29% for a QDSSCs based on (001)-TiO2 nanosheets, 2.18% for a QDSSCs based on (100)-TiO2 nanosheets, and 1.46% for a QDSSCs based on Degussa P25. The improved photoelectric conversion efficiency of QDSSCs based on TiO2 nanosheets photoanode is mainly attributed to more CdS QDs loading beneficial from higher surface area, the reduction of electron recombination and the enhancement of electron transport. Recently, Zhong's group developed a linker-assisted deposition method for fabricating QDSSCs, the postsynthesis colloidal quantum dots bound tightly on the TiO2 photoanodes via the carboxylate linkage of bifunctional linker molecule capped on the quantum dots.10 The carboxylate linkage offers the opportunity for the QD sensitizer to behave like the organic dye with intimate interfacial contact with TiO2, and due to the simple sensitized method and high quality QDs, an exiting certified PCE (11.60%) of QDSSCs based the colloidal QDs (ZnCuInSe) has been obtained, which indicate that the post synthesis colloidal QDs capped with bifunctional linker molecule have significant potential for obtaining high efficiency QDSSCs.10 As mentioned above, the post synthesis colloidal QDSSCs have big size (∼5 nm), so the photoanode sensitized by post synthesis colloidal QDs is very different with the photoanode sensitized by Dye and in situ growth method of quantum dots. For obtaining high efficiency QDSSCs, it is very urgent and necessary to seeking for the suitable photoanode with high absorption abilities for the postsynthesis colloidal QDs, which will lead to better photoelectric conversion efficiency of cells. Both theoretical and experimental studies have shown that the (001) surface of anatase TiO2 is much more reactive than the thermodynamic stable (101) crystal plane, which may be the dominant source of active sites, and the anatase (001) surfaces with high surface energy have strong ability to absorb dissociatively (COOH) group.5,28–30,34 Herein, the colloidal CdSe QDs sensitized solar cells based on the anatase TiO2-NSs photoanode have been studied in this paper, the experimental results indicates that the anatase TiO2-NSs photoanode with exposed (101) crystal planes is a optimized candidate for obtaining high efficiency QDSSCs.
2. Experimental section
2.1. Material and synthesis
Selenium power (99.99%), cadmium oxide (99.99%), 1-octadecene (90%), oleylamine (OAM), trioctylphosphine (90%), ethyl cellulose (EC), and terpineol were purchased from Aldrich. Oleic acid (OA, 90%) and 3-mercaptopropionic acid (MPA, 99%) were obtained from Alfa. Titanium(IV) t-butoxide and hydrofluoric acid were purchased from Sinopharm Chemical Reagent Company Limited. All chemicals were used as received without further purification.Anatase TiO2-NSs with dominant (001) crystal planes were prepared by a facile one-pot hydrothermal synthesis: a precursor solution containing 30 mL of Ti(OC4H9)4 and 4 mL of hydrofluoric acid (with a concentration of 40 wt%) was put in a Teflon-lined autoclave at 180 °C for 24 h. After hydrothermal reaction, the white precipitate was collected, washed three times with ethanol and distilled water, and finally dried in an oven at 80 °C for 12 h. To check the performance of TiO2-NSs in the QDSSCs, according to the report,28 TiO2-NPs with dominant (101) crystal planes are also prepared under the same conditions except that the hydrofluoric acid was replaced by distilled water (4 mL) for comparison. The above samples were annealed at 450 °C for 30 min.
2.2. Fabrication of QDSSCs
For preparing the mesoporous TiO2 photoanodes pastes for QDSSCs, an amount of 0.5 g of TiO2 powder was added to 3.8 mL of anhydrous ethanol in a round-bottomed flask, and sonicated for 10 min, then, 2 g α-terpineol and 2.6 g of an ethanol solution with 10 wt% EC were dispersed and sonicated in the mixture consecutively. Finally, the ethanol was removed via a rotary-evaporator.The fabrication of the mesoporous TiO2 photoanode films: firstly, the fluorine-doped tin oxide (FTO) glass was soaked into 40 mM TiCl4 solution and stored in an oven at 70 °C for 30 min in a close vessel and washed with water and ethanol. Secondly, the prepared TiO2 pastes were deposited on the FTO glass by a doctor blade method, followed by gradually drying at 120 °C for 7 min each time to get an appropriate thickness. Finally, the electrode was gradually heated under an airflow oven at 325 °C for 5 min, at 375 °C for 5 min, and at 450 °C for 15 min, and finally at 500 °C for 15 min.
Synthesis process of the water-soluble mercaptopropionic (MPA)-capped CdSe QDs refers to the reports.35 The obtained mesoporous TiO2 photoanode films were soaked in the pre-prepared water-soluble MPA-capped CdSe QDs aqueous solution for 4 h. An inorganic ZnS/SiO2 double barrier coating layer was employed to treat the surface of the QD-sensitized photoanode. Compared with the ZnS single coating layer, ZnS/SiO2 double coating layer acts as a more effective energy barrier to suppress recombination of photogenerated electrons in the quantum dots with holes residing in electrolyte.36 The detail ZnS/SiO2 double barrier coating process is following: the CdSe-sensitized TiO2 films were alternatively immersed in Zn(OAc)2 and Na2S aqueous solutions 4 times. After coating ZnS layer, further SiO2 coating was carried out by dipping the ZnS coated photoanode films in 0.01 M tetraethylorthosilicate ethanol solution containing 0.1 M NH4OH for 2 h and then rinsed with water and dried in air. Finally, the TiO2 photoanode was sintered at 300 °C for 2 min to obtain the expected photoanode films. The Cu2S counter electrode was prepared by immersing brass in HCl solution at 70 °C for 10 min and rinsed with deionized water and ethanol, then blown dry using nitrogen gas, and subsequently dipping it into polysulfide solution for 10 min.
The cells were assembled by sandwiching the CdSe-sensitized TiO2 photoanode and the Cu2S counter electrode together with a polysulfide electrolyte composed of 2.0 M S, 2.0 M Na2S and 0.2 M KCl aqueous solution in the interspace.
2.3. Characterization
The morphology and the crystal structure of the samples were investigated by field-emission scanning electron microscopy (FE-SEM; SU70, Hitachi, Japan), transmission electron microscopy (TEM; FEI, Tecnai TF20) and X-ray diffraction (XRD; D/max2600, Rigaku, Japan). An energy dispersive X-ray (EDX) spectroscope coupled to a FE-SEM was used to analyze the composition of the samples. The specific surface areas and the pore size distribution of the samples were measured with a Micromeritics ASAP 2010 instrument and analyzed by the Brunauer–Emmett–Teller (BET) method. The light scattering and absorption properties of the samples were investigated by ultraviolet-visibles spectrophotometer (Perkin–Elmer, Lambda 850). Current–voltage curves of the QDSSCs were characterized using a Keithley 2400 sourcemeter using a solar light simulator (Zolix SS150) to simulate AM 1.5G illumination (100 mW cm−2). The intensity was calibrated with a NREL-calibrated silicon solar cell (Zolix QE-B1). The incident photon-to-current conversion efficiency (IPCE) spectra were measured by a DCS300PA incorporated with a Spectral Product LSP-X150 monochromator. The electrochemical impedance spectroscopy (EIS) spectra were measured by an electrochemical workstation (VMP3, France) at a bias potential of −0.6 V with a frequency range from 100 mHz to 100 kHz under dark conditions. Intensity-modulated photovoltage spectroscopy (IMVS) and intensity-modulated photocurrent spectroscopy (IMPS) measurements (Zahner Elektrik, Germany) were carried out on the electron lifetime and the electron transport time. A white light-emitting diode (wlr-01) was used as the light source. The frequency range was 0.1–1000 Hz.3. Results and discussion
Fig. 1a and b show the SEM and TEM images of the TiO2-NSs, which show clearly that the prepared nanosheets consist of rectangular sheet-shaped structures, an side length of ca. 30–70 nm and the thickness of ca. 5.7 nm (Fig. 1a, b and d). The HRTEM image (Fig. 1d) shows clearly that the lattice spacing parallel to the top and bottom crystal planes is ca. 0.235 nm, corresponding to the (001) planes of anatase TiO2, which indicates the top and bottom crystal planes of the TiO2-NS are the (001) and (00![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) planes, respectively. On the basis of the above SEM and TEM results, we can roughly calculate that the percentage of exposed (001) crystal planes is more than 71% on the TiO2-NSs (the calculation process can be found in ESI†). Fig. 1f shows a typical HRTEM image taken from the TiO2-NPs. As reported in the literature,28,29 the fringe spacing of 0.35 nm corresponds to the (101) crystal planes of anatase TiO2, and the percentage of exposed (001) crystal planes for the TiO2-NPs is usually less than 10%.28,37
) planes, respectively. On the basis of the above SEM and TEM results, we can roughly calculate that the percentage of exposed (001) crystal planes is more than 71% on the TiO2-NSs (the calculation process can be found in ESI†). Fig. 1f shows a typical HRTEM image taken from the TiO2-NPs. As reported in the literature,28,29 the fringe spacing of 0.35 nm corresponds to the (101) crystal planes of anatase TiO2, and the percentage of exposed (001) crystal planes for the TiO2-NPs is usually less than 10%.28,37
 | ||
| Fig. 1 SEM (a), TEM (b) and HRTEM (c and d) images of TiO2-NSs as well as TEM (e) and HRTEM (f) images of TiO2-NPs. | ||
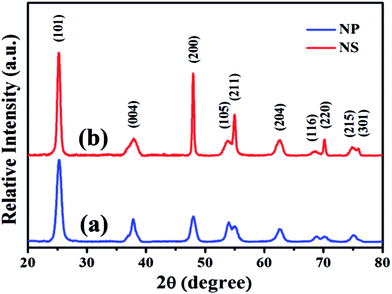
The phase structure, crystalline size, and crystallinity of TiO2 have great effect on its photoelectric conversion efficiency of QDSSCs. Fig. 2 gives the XRD patterns of the as-prepared TiO2 samples. As can be seen from the XRD patterns, all identified peaks can be perfectly indexed to anatase TiO2 (JCPDS card no. 21-1272). The well defined and sharp Bragg peaks indicate good crystallinity of the samples. Further observation displays that TiO2-NSs show sharper diffraction peaks than TiO2-NPs, indicating its better crystallization and larger crystallites due to the enhanced effect of F−.38 Meanwhile, the anatase crystallite sizes can be calculated according to the Scherrer formula: D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, D is the average crystal size, K is the Scherrer constant and equal to 0.89, λ is the wavelength of the X-ray radiation and equal to 0.15418 nm, β is the full width at half-maximum (FWHM) of the (101) plane for the anatase TiO2 samples, and θ is the diffraction angle. The FWHM of the (101) plane and average crystalline sizes of TiO2-NSs and TiO2-NPs samples were listed in Table S2.† It can be seen that the sample of TiO2-NSs has larger average crystalline size and better crystallinity.
θ, D is the average crystal size, K is the Scherrer constant and equal to 0.89, λ is the wavelength of the X-ray radiation and equal to 0.15418 nm, β is the full width at half-maximum (FWHM) of the (101) plane for the anatase TiO2 samples, and θ is the diffraction angle. The FWHM of the (101) plane and average crystalline sizes of TiO2-NSs and TiO2-NPs samples were listed in Table S2.† It can be seen that the sample of TiO2-NSs has larger average crystalline size and better crystallinity.
The specific surface areas and pore size distributions of the TiO2-NPs and TiO2-NSs samples were characterized using nitrogen adsorption–desorption isotherms and the results were shown in Fig. 3, all samples have isotherms of type IV according to Brunauer–Deming–Deming–Teller (BDDT) classification.28 For the TiO2-NPs, the hysteresis loop shows type H2, and the pore size distribution (inset in Fig. 3) calculated from the desorption branch of the nitrogen isotherm by the BJH method shows a wide range from 5 to 50 nm with a maximum pore diameter of about 7.8 nm. For the sample of TiO2-NSs, the shapes of the hysteresis loops is between H2 and H4, indicating the presence of slit-like pores. The corresponding pore size distribution curve (inset in Fig. 3) shows a maximum pore diameter of about 12.4 nm. It is obvious that the pore size increases and BET specific surface areas decrease in TiO2-NSs (85 m2 g−1) compared with TiO2-NPs (133 m2 g−1), due to growth of TiO2 crystallites (see XRD and TEM results). Although the BET specific surface area of the TiO2-NSs is a little lower than the TiO2-NPs, their large pore size distribution would be extremely useful for QDSSCs, which will provide efficient pathways for the filling of colloidal QDs and electrolyte molecules. Moreover, low specific surface area results in fewer surface traps (recombination centers), which will reduce the charge recombination.
 | ||
| Fig. 3 Nitrogen adsorption–desorption isotherms and the corresponding pore size distribution curves (inset) of the TiO2-NSs and NPs. | ||
The as-synthesized TiO2-NPs and TiO2-NSs were fabricated into viscous pastes, respectively, and then screen-printed onto the FTO glass, further used as the photoanodes in QDSSCs. The surface and cross-sectional morphology of the TiO2-NSs film and the TiO2-NPs film were characterized by SEM. Fig. 4a and b show the surface morphology of the TiO2-NSs film and the TiO2-NPs film, respectively. It is obvious that the surface of TiO2-NSs film is flat, uniform, and crack-free compared with that of TiO2-NPs film. Meanwhile, compared with the photoanode of TiO2-NPs film, there are lots of relatively uniform large pore on the surface of the photoanode based on the TiO2-NSs, it would provide many efficient pathways for the filling of quantum dots and electrolyte molecules in QDSSCs, which in turn helped to maximizes loading of QDs and regenerate the QDs, being very important to improve performance of the QDSSCs. Fig. 4c shows that the thickness of the TiO2-NSs film was about 11 μm, which is similar to that of the TiO2-NPs film (Fig. 4d). Further observation indicates that the TiO2-NSs film with no obvious boundaries was observed between each printing layers, which is favorable for electron transfer resulting in a low charge recombination rate.
 | ||
| Fig. 4 Surface (a and b) and cross-sectional (c and d) SEM images of the TiO2-NSs and NPs photoanode films, respectively. | ||
To investigate the light scattering property of the photoanodes between TiO2-NSs film and TiO2-NPs film, the UV/Vis reflectivity spectra of the photoanodes were shown in Fig. 5a. The TiO2-NSs film exhibits slightly higher reflectance than the TiO2-NPs film only from the 350 nm to 440 nm, which reveal that the TiO2-NSs film hardly improves light scattering compared with the TiO2-NPs film. Fig. 5b shows the optical absorbance of the colloidal CdSe QDs sensitized photoanodes (TiO2-NSs/CdSe, TiO2-NPs/CdSe) based on TiO2-NSs film and TiO2-NPs film, respectively. It can be seen that the absorbance of the TiO2-NSs/CdSe film is much higher than that of TiO2-NPs/CdSe film, indicating that more amount of QDs is absorbed on the TiO2-NSs photoanode. As shown in the inset of Fig. 5b, much darker color of the TiO2-NSs film compared to that of TiO2-NPs film also implies the more amounts of QDs adsorbed onto the former than the latter. To further investigate the difference of colloidal CdSe QDs-loading on the TiO2-NSs film and TiO2-NPs film, the atomic ratios of Cd/Ti in different depth of the TiO2-NSs/CdSe and TiO2-NPs/CdSe films are shown in Fig. 5c. Fig. S1† shows the detail EDX images in the different depth sections of TiO2-NSs/CdSe and TiO2-NPs/CdSe films. The atomic ratios of Cd/Ti shows slightly reduction with the increasing of the depth in the two photoanodes, which could be because the upper pores of the film are blocked by the QDs, and thus suppressing the filling of CdSe QDs in the interior of the TiO2 film. Meanwhile, it can be clearly seen, the atomic ratios of Cd/Ti in TiO2-NSs/CdSe film is about 3 times higher than that in TiO2-NPs/CdSe film at any depth, which confirms that the more amounts of QDs adsorbed onto the TiO2-NSs film compared with the CdSe/TiO2-NPs flim. The superior QDs loading ability of 2D anatase TiO2-NSs with exposed (001) crystal planes should be mainly attributed to the two factors, the anatase (001) surfaces with high surface energy have strong ability to absorb dissociatively (COOH) group, large pore size of TiO2-NSs film is beneficial for filling of quantum dots. A schematic diagram of the colloidal CdSe-QDs absorbed on the TiO2 NS and the NP by the assistance of the carboxylate linker of the bifunctional linker molecule (MPA) is shown in Fig. 5d. To check the performance of QDSSCs based TiO2-NSs/CdSe and TiO2-NPs/CdSe films, respectively, then, they are assembled into QDSSCs.
Comparison of the current density–voltage (J–V) curves of the two QDSSCs under one full illumination (100 mW cm−2) is shown in Fig. 6a, and detailed parameters of the two solar cells are summarized in Table S3.† It shows obviously that the cell based on the TiO2-NSs has better photo-electric conversion efficiency than that based on TiO2-NPs, and an impressive PCE as high as 5.01% has been achieved, which is 63% higher than that based on TiO2 nanoparticle film (3.07%). In detail, the Jsc increased from 12.60 mA cm−2 to 16.95 mA cm−2, the FF increased from 0.42 to 0.50 and the Voc increased from 582 mV to 591 mV. In order to further illustrate the effect of the TiO2-NSs film and TiO2-NPs film on the performance of QDSSCs, the monochromatic incident photon to current conversion efficiency (IPCE) spectra were measured as a function of incident photon wavelength from 375 nm to 700 nm (as shown in Fig. 6b), and the inset shows the UV-Vis absorption spectra of CdSe QDs. An obvious enhancement in quantum efficiency can be found over the whole test spectral range. Usually, the IPCE value of solar cell can be defined as IPCE(λ) = LHE(λ)φinj(λ)φregηcc(λ). Where the LHE(λ) is light-harvesting efficiency, φinj(λ) and φreg are the efficiency of electron injection and quantum dots regeneration, respectively, the ηcc(λ) is the electron collection efficiency.1 LHE(λ) is mainly determined by the loading amount of sensitizers and the light scattering ability of the photoanode films. In our case, the similar refection spectra have been observed in the two photoanode films, and the TiO2-NPs photoanode films show a little better reflection ratio than TiO2-NSs from 400 nm to 700 nm. Therefore, we can conclude the enhancement of the LHE of the photoanode films based on the TiO2-NSs is due to the significant quantum dots loading, which is the dominant responsibility for the enhancement in Jsc. The absorption spectra and EDX date further constituted proof of the enhancement of quantum dots loading in the TiO2-NSs photoanode films.
 | ||
| Fig. 6 (a) J–V characteristics and (b) IPCE spectra of QDSSCs based on TiO2-NPs and TiO2-NSs photoanode films. Inset, UV-Vis absorption spectra of CdSe QDs. | ||
In order to investigate the interfacial electron transfer resistance, the electrochemical impedance spectra (EIS) of the two cells were also measured, shown in Fig. 7a. The Nyquist curves exhibited two semicircles which corresponded to the electron injection at the counter electrode/electrolyte interface and transfer in the electrolyte at high frequencies (R1, the first semicircle), and recombination resistance for the electron-transfer process at the TiO2 films/QDs/electrolyte interface and transport in the TiO2 film (R2, the second semicircle). The fitting results of the charge transfer resistance within the two cells are summarized in Table S4.† Apparently, the R2 of TiO2 NS photoanode increases from 61.39 Ω to 144.52 Ω compared with TiO2 NP photoanode, which predicted the reduced interfacial recombination, and thus leading to higher Voc. The increase of R2 is mainly attributed to the reduction of the contact area of TiO2 with the electrolyte, because the relatively low specific surface area and high loading of QDs on the TiO2 NSs photoanode impede the direct exposure of TiO2 to the electrolyte. Furthermore, it has been illustrated that Rs corresponded to the sheet resistance of the FTO glass substrate and the contact resistance at the FTO/TiO2 interface. The Rs values of QDSSCs based on TiO2-NSs and TiO2-NPs photoanodes calculated according to the equivalent circuit were 14.79 Ω and 16.35 Ω, respectively. It is clear that TiO2-NSs photoanode has the smaller Rs, implying better electronic contact between nanosheets and FTO glass due to better crystallinity and less defects existing in TiO2 nanosheets than in nanoparticles, which is beneficial for a higher FF.39–41 To further characterize the electron transport and charge recombination within the two TiO2 films, the intensity-modulated photocurrent spectroscopy (IMPS) and intensity-modulated photovoltage spectroscopy (IMVS) measurements were carried out under a modulated light emitting diodes (605 nm) with a series of different light intensity (60, 90 and 120 W m−2). The electron transport time (τd) and the electron lifetime (τr) can be estimated from the IMPS and IMVS plots respectively by using the following expressions: τd = 1/2πfIMPS, τr = 1/2πfIMVS, where the fIMPS or fIMVS is the characteristic minimum frequency of the IMPS and IMVS imagery component. Fig. 7b shows the τd and the τr of the QDSSCs based on different photoanodes as a function of various light intensities. Both τd and τr decrease with the increasing of light intensity due to the fact that deep traps are filled at higher light intensity and electron trapping/detrapping involves shallower levels, and hence electron transfer becomes faster. Specifically, longer τr and lower τd are obtained in the TiO2-NSs based cell than that of TiO2-NPs based cell, indicating a slower recombination rate and a faster transport rate in the TiO2-NSs photoanode film. The performances will be attributed to the fact: in the TiO2-NSs photoanode film, the large particle size and high crystallinity provide the shorter and efficient pathway for electron transfer, thus leading to the enhanced electron transport in the cell; moreover, the smaller specific surface area would decrease additional recombination centers to suppress charge recombination, leading to prolonged electron lifetime, and thus enhancing charge collection efficiency, which is in good agreement of above measurements.
 | ||
| Fig. 7 (a) Electrochemical impedance spectroscopy and (b) IMPS/IMVS tests of QDSSCs based on TiO2 NSs and NPs photoanode films. | ||
4. Conclusions
In summary, anatase TiO2 nanosheets and nanoparticles have been synthesized by a facile one-pot hydrothermal method with the presence and absence of HF, respectively. Then, the photoelectric conversion efficiency of CdSe sensitized solar cell based on TiO2 NSs and NPs photoanodes are compared under the same condition and the same film thickness, and their photoelectric conversion efficiencies are 5.01% and 3.08%, respectively. The considerable improvement of power conversion efficiency can be mainly attributed to superior CdSe quantum dots loading on the anatase TiO2-NSs with exposed (001) crystal planes due to the large pore size of the photoanode and the strong ability of a amount of high energy (001) crystal planes exposed to absorb dissociatively (COOH) group, leading to enhanced light harvesting. Furthermore, the good crystallization, large particle size and low specific surface area of the TiO2-NSs result in fewer defects and provide efficient electron transfer pathways and prolong electron lifetime. Meanwhile, the large pore size of TiO2-NSs photoanode will diminish the infiltration resistance of the electrolyte and make the substance exchange unhindered, which in turn helped to regenerate the QD. This work provides an optimal candidate for obtaining high efficiency QDSSCs.Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant No. 61404039), Heilongjiang Province Foundation for Returned Chinese Scholars (Grant No. LC201401), Educational Commission of Heilongjiang Province of China (Grant No. 12531Z006). The work was also supported by a grant from technology project of returned overseas students of Heilongjiang Province.References
- Y. F. Xu, W. Q. Wu, H. S. Rao, H. Y. Chen, D. B. Kuang and C. Y. Su, Nano Energy, 2015, 11, 621–630 CrossRef CAS.
- J. J. Tian and G. Z. Cao, Nano Rev., 2013, 4, 22578 Search PubMed.
- J. J. Lin, A. Nattestad, H. Yu, Y. Bai, L. Z. Wang, S. X. Dou and J. H. Kim, J. Mater. Chem. A, 2014, 2, 8902–8909 CAS.
- P. F. Cheng, S. S. Du, Y. X. Cai, F. M. Liu, P. Sun, J. Zheng and G. Y. Lu, J. Phys. Chem. C, 2013, 117, 24150–24156 CAS.
- X. Wu, Z. G. Chen, G. Q. (Max) Lu and L. Z. Wang, Adv. Funct. Mater., 2011, 21, 4167–4172 CrossRef CAS.
- U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissortel, J. Salbeck, H. Spreitzer and M. Grätzel, Nature, 1998, 395, 583–585 CrossRef CAS.
- E. Y. Guo, L. W. Yin and L. Y. Zhang, CrystEngComm, 2014, 16, 3403 RSC.
- H. S. Rao, W. Q. Wu, Y. Liu, Y. F. Xu, B. X. Chen, H. Y. Chen, D. B. Kuang and C. Y. Su, Nano Energy, 2014, 8, 1–8 CrossRef CAS.
- M. C. Hanna and A. J. Nozik, J. Appl. Phys., 2006, 100, 074510 CrossRef.
- J. Du, Z. L. Du, J. S. Hu, Z. X. Pan, Q. Shen, J. K. Sun, D. H. Long, H. Dong, L. T. Sun, X. H. Zhong and L. J. Wan, J. Am. Chem. Soc., 2016, 138, 4201–4209 CrossRef CAS PubMed.
- J. Wang, Y. Li, Q. Shen, T. Izuishi, Z. X. Pan, K. Zhao and X. H. Zhong, J. Mater. Chem. A, 2016, 4, 877–886 CAS.
- J. J. Tian, R. Gao, Q. F. Zhang, S. E. Zhang, Y. W. Li, J. Lan, X. H. Qu and G. Z. Cao, J. Phys. Chem. C, 2012, 116, 18655–18662 CAS.
- J. Wang, I. Mora-Seró, Z. X. Pan, K. Zhao, H. Zhang, Y. Y. Feng, G. Yang, X. H. Zhong and J. Bisquert, J. Am. Chem. Soc., 2013, 135, 15913–15922 CrossRef CAS PubMed.
- J. Du, X. X. Meng, K. Zhao, Y. Li and X. H. Zhong, J. Mater. Chem. A, 2015, 3, 17091–17097 CAS.
- C. Chen, M. D. Ye, N. Zhang, X. R. Wen, D. J. Zheng and C. J. Lin, J. Mater. Chem. A, 2015, 3, 6311–6314 CAS.
- R. Zhou, Q. F. Zhang, E. Uchaker, J. Lan, M. Yin and G. Z. Cao, J. Mater. Chem. A, 2014, 2, 2517–2525 CAS.
- J. Y. Xiao, Q. L. Huang, J. Xu, C. H. Li, G. P. Chen, Y. H. Luo, D. M. Li and Q. B. Meng, J. Phys. Chem. C, 2014, 118, 4007–4015 CAS.
- M. Grätzel, Nature, 2001, 414, 338–344 CrossRef PubMed.
- S. M. Wang, W. W. Dong, X. D. Fang, S. Z. Wu, R. H. Tao, Z. H. Deng, J. Z. Shao, L. H. Hu and J. Zhu, J. Power Sources, 2015, 273, 645–653 CrossRef CAS.
- R. Zhou, H. H. Niu, Q. F. Zhang, E. Uchaker, Z. Q. Guo, L. Wan, S. D. Miao, J. Z. Xu and G. Z. Cao, J. Mater. Chem. A, 2015, 3, 12539–12549 CAS.
- H. L. Feng, W. Q. Wu, H. S. Rao, Q. Wan, L. B. Li, D. B. Kuang and C. Y. Su, ACS Appl. Mater. Interfaces, 2015, 7, 5199–5205 CAS.
- H. L. Feng, W. Q. Wu, H. S. Rao, L. B. Li, D. B. Kuang and C. Y. Su, J. Mater. Chem. A, 2015, 3, 14826–14832 CAS.
- K. S. Leschkies, R. Divakar, J. Basu, E. Enache-Pommer, J. E. Boercker, C. B. Carter, U. R. Kortshagen, D. J. Norris and E. S. Aydil, Nano Lett., 2007, 7, 1793–1798 CrossRef CAS PubMed.
- Y. L. Liao, J. Zhang, W. G. Liu, W. X. Que, X. T. Yin, D. N. Zhang, L. H. Tang, W. D. He, Z. Y. Zhong and H. W. Zhang, Nano Energy, 2015, 11, 88–95 CrossRef CAS.
- T. Toyoda and Q. Shen, J. Phys. Chem. Lett., 2012, 3, 1885–1893 CrossRef CAS PubMed.
- B. K. Liu, Y. J. Sun, X. S. Wang, L. J. Zhang, D. J. Wang, Z. W. Fu, Y. H. Lin and T. F. Xie, J. Mater. Chem. A, 2015, 3, 4445–4452 CAS.
- J. J. Tian, Q. F. Zhang, L. L. Zhang, R. Gao, L. F. Shen, S. E. Zhang, X. H. Qu and G. Z. Cao, Nanoscale, 2013, 5, 936–943 RSC.
- J. G. Yu, J. J. Fan and K. L. Lv, Nanoscale, 2010, 2, 2144–2149 RSC.
- W. G. Wang, H. Y. Zhang, R. Wang, M. Feng and Y. M. Chen, Nanoscale, 2014, 6, 2390–2396 RSC.
- W. G. Yang, J. M. Li, Y. L. Wang, F. Zhu, W. M. Shi, F. R. Wan and D. S. Xu, Chem. Commun., 2011, 47, 1809–1811 RSC.
- T. You, L. Jiang, K. L. Han and W. Q. Deng, Nanotechnology, 2013, 24, 245401 CrossRef PubMed.
- J. D. Peng, P. C. Shih, H. H. Lin, C. M. Tseng, R. Vittal, V. Suryanarayanan and K. C. Ho, Nano Energy, 2014, 10, 212–221 CrossRef CAS.
- W. W. Sun, T. Peng, Y. M. Liu, W. J. Yu, K. Zhang, H. F. Mehnane, C. H. Bu, S. S. Guo and X. Z. Zhao, ACS Appl. Mater. Interfaces, 2014, 10, 9144 Search PubMed.
- A. Selloni, Nat. Mater., 2008, 7, 613–615 CrossRef CAS PubMed.
- H. Zhang, K. Cheng, Y. M. Hou, Z. Fang, Z. X. Pan, W. J. Wu, J. L. Hua and X. H. Zhong, Chem. Commun., 2012, 48, 11235–11237 RSC.
- K. Zhao, Z. X. Pan, I. Mora-Seró, E. Canovas, H. Wang, Y. Song, X. Q. Gong, J. Wang, M. Bonn, J. Bisquert and X. H. Zhong, J. Am. Chem. Soc., 2015, 137, 5602–5609 CrossRef CAS PubMed.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638–642 CrossRef CAS PubMed.
- J. T. Jiu, S. Isoda, F. M. Wang and M. Adachi, J. Phys. Chem. B, 2006, 110, 2087–2092 CrossRef CAS PubMed.
- J. R. Jennings, Y. R. Liu, F. Safari-Alamuti and Q. Wang, J. Phys. Chem. C, 2012, 116, 1556–1562 CAS.
- Q. Wang, S. Ito, M. Grätzel, F. Fabregat-Santiago, I. Mora-Seró, J. Bisquert, T. Bessho and H. Imai, J. Phys. Chem. B, 2006, 110, 25210–25221 CrossRef CAS PubMed.
- J. V. D. Lagemaat, N. G. Park and A. J. Frank, J. Phys. Chem. B, 2000, 104, 2044–2052 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: EDX images, comparison of physical properties of TiO2-NSs, TiO2-NPs, detailed photovoltaic parameters of QDSSCs based on different photoanodes and detailed simulative value from EIS spectra. See DOI: 10.1039/c6ra10628e |
| This journal is © The Royal Society of Chemistry 2016 |