Complex impedance, dielectric properties and electrical conduction mechanism of La0.5Ba0.5FeO3−δ perovskite oxides
Abstract
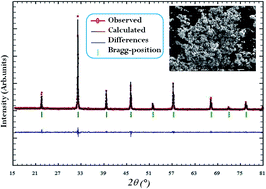
The new perovskite compound La0.5Ba0.5FeO3−δ was prepared by the sol–gel method. The Rietveld refinement, from the X-ray diffraction pattern, proved that the sample presents single phase type cubic structure at room temperature. In addition, electrical properties were performed using complex impedance spectroscopy (CIS) technique as a function of frequency (100 Hz to 7 MHz) at various temperatures (278–573 K). As a matter of fact, impedance analysis was performed using the equivalent circuit model and it indicated the presence of a single semicircle arc. Furthermore, the obtained results of the AC conductivity have been discussed in terms of the non-overlapping small polaron tunneling (NSPT) model. Finally, it is worth mentioning that the conductivity of our investigated perovskite oxide was found to be σ573 = 1.23 × 10−2 Ω−1 cm−1. These results confer to this material a potential application as a cathode material.

 Please wait while we load your content...
Please wait while we load your content...