Aggregation and stabilization of multiwalled carbon nanotubes in aqueous suspensions: influences of carboxymethyl cellulose, starch and humic acid†
Abstract
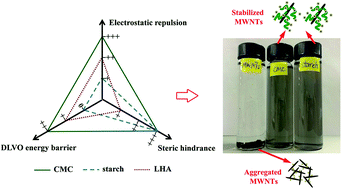
Aggregation and stability of multiwalled carbon nanotubes (MWNTs) in aqueous solutions were investigated in the presence of two polysaccharide stabilizers (carboxymethyl cellulose (CMC) and a water soluble starch) and a natural organic matter (leonardite humic acid (LHA)). While all stabilizers inhibited aggregation of MWNTs, the stabilization effectiveness ranked as CMC > starch > LHA. In the presence of 0.06 wt% of CMC or 0.08 wt% of starch, 10 mg L−1 of MWNTs were fully stabilized (no gravity settling). At 10 mg L−1 of MWNTs, the addition of 5 mg L−1 as total organic carbon of CMC increased the critical coagulation concentration (CCC) of MWNTs from ∼25 to ∼210 mM in NaCl solution and from ∼0.9 to ∼2.6 mM in CaCl2 solution. The three stabilizers showed very different effects on the electrophoretic mobility (EPM) of MWNTs: the coating of negatively charged CMC enhanced EPM from −3.24 × 10−8 m2 V−1 s−1 for bare MWNTs to −5.22 × 10−8 m2 V−1 s−1, while the coating of neutral starch slightly curbed EPM to −2.24 × 10−8 m2 V−1 s−1, and LHA hardly affected EPM. Derjaguin–Landau–Verwey–Overbeek (DLVO) theory can interpret the stabilization mechanisms, which reveals that CMC stabilizes MWNTs through enhanced electrostatic repulsion, primary energy barrier and steric hindrance, whereas starch and LHA work primarily through steric hindrance. CMC and starch exert greater steric hindrance than LHA, partially due to the long chains of the polysaccharides and the associated steric hindrance. The information can facilitate environmental applications of carbon nanotubes and improve our understanding of the environmental fate and transport of engineered stabilized nanomaterials.

 Please wait while we load your content...
Please wait while we load your content...