Ordered mesoporous crystalline titania with high thermal stability from comb-like liquid crystal block copolymers†
Abstract
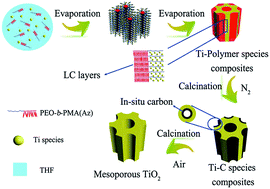
Ordered mesoporous crystalline titania with high thermal stability is directly synthesized in a simple one-pot procedure via an integrated approach of a solvent evaporation-induced self-assembly (EISA) technique and template-carbonization strategy by using a comb-like amphiphilic liquid crystal (LC) block copolymer (BCP) PEO-b-PMA(Az) with abundant sp2-carbons as a structure-directing agent. These BCP containing LC rigid segments with distinct hydrophilic/hydrophobic contrast can form robust micelles and co-assemble with titania sol into ordered mesostructures which are stable during the whole EISA process. Template carbonization in nitrogen after ageing is the key step to obtain the ordered crystalline mesostructures. The synthesized titania materials have a large pore size (∼4.87 nm) and pore volume (∼0.37 cm3 g−1) and high surface area (∼239 m2 g−1), as well as high thermal stability (>700 °C). Additionally, mesoporous titania shows good photocatalytic activity for the degradation of rhodamine B in an aqueous suspension due to its highly crystalline framework and large surface area. This simple, yet facile method can therefore be expanded to other ordered crystalline mesoporous materials through high-temperature heat treatments.


 Please wait while we load your content...
Please wait while we load your content...