Effect of RGO deposition on chemical and mechanical reliability of Ag nanowire flexible transparent electrode†
B. Hwang‡
ab,
M. Park‡a,
T. Kima and
S. M. Han*a
aGraduate School of Energy Environment Water and Sustainability, Korea Advanced Institute of Science & Technology, Daejeon, 305701, Republic of Korea. E-mail: smhan01@kaist.ac.kr
bBASF Electronic Materials R&D Center Asia, Suwon, 440746, Republic of Korea
First published on 8th July 2016
Abstract
Graphene is known to prevent permeation of gases that can effectively prevent oxidation of Ag nanowires. In this study, the role of the reduced graphene oxide (RGO) in chemical stability and its effect on the mechanical reliability was studied for a Ag nanowire/RGO hybrid transparent electrode. Bending fatigue tests up to 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles were performed by monitoring the in situ resistance change while imposing fixed, uniform bending strain on Ag nanowire networks with and without a RGO layer. A thin layer of RGO with an optimized thickness of ∼0.8 nm deposited on the Ag nanowire networks sustained excellent reliability of the Ag nanowire networks, where the fractional resistance increase was 2.7% after 800
000 cycles were performed by monitoring the in situ resistance change while imposing fixed, uniform bending strain on Ag nanowire networks with and without a RGO layer. A thin layer of RGO with an optimized thickness of ∼0.8 nm deposited on the Ag nanowire networks sustained excellent reliability of the Ag nanowire networks, where the fractional resistance increase was 2.7% after 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Furthermore, adopting the RGO layer significantly lowered the oxidation of Ag nanowires, and the bending fatigue properties after exposure to ambient air for 132 h at 70 °C indicated remarkable enhancement due to suppression of the oxide formation on the surface of the Ag nanowires. Lastly, a highly reliable Ag nanowire/RGO hybrid electrode was fabricated using mechanical welding by subjecting it to bending strain in order to form localized junctions without having to go through any post annealing processes.
000 cycles. Furthermore, adopting the RGO layer significantly lowered the oxidation of Ag nanowires, and the bending fatigue properties after exposure to ambient air for 132 h at 70 °C indicated remarkable enhancement due to suppression of the oxide formation on the surface of the Ag nanowires. Lastly, a highly reliable Ag nanowire/RGO hybrid electrode was fabricated using mechanical welding by subjecting it to bending strain in order to form localized junctions without having to go through any post annealing processes.
1. Introduction
With the technological developments in flexible displays, there is an increasing demand for suitable flexible transparent electrodes.1–7 Indium tin oxide (ITO) is the most widely used material for displays owing to its high optical transmittance in the visible range and low sheet resistance. However, ITO has several limitations including the scarcity and high cost of indium, high processing temperature that is unsuitable for polymeric substrates, and the intrinsic brittleness that results in brittle fracture after 1% bending strain.8,9 Therefore, research to develop a new material to replace ITO is currently underway. Potential candidates that can overcome the limitations of ITO include metal nanowires,6,7 single-walled carbon nanotubes (CNT),2,3 and graphenes.4,5 Among these candidates, the Ag nanowire network electrode is especially promising compared to the carbon based materials due to its high conductivity and low cost synthesis methods.6,10,11 In addition, the high flexibility of the Ag nanowire networks makes this a viable technology for commercial flexible display applications.12The major drawback of the Ag nanowire network as a transparent electrode, however, is that the Ag nanowires are easily oxidized when exposed to moist air, which results in the increase in sheet resistance of the Ag nanowire network.13–15 Therefore, a suitable protective layer is needed to suppress the oxidation of the Ag nanowires. Reduced graphene oxide (RGO) is an attractive candidate for the protective layer owing to its low permeability to gas or H2O molecules16 and excellent optical transmittance.17 A few studies so far have reported the hybrid transparent electrode of RGO layer on the surface of the Ag nanowire networks, where a remarkable chemical stability under ambient moisture without significant loss of optical properties were demonstrated.13,15,17–19
An important remaining issue in applying the Ag nanowire/RGO hybrid electrode for flexible electronics is the mechanical reliability as the RGO layer can degrade the previously reported excellent flexibility and reliability of the Ag nanowire network.12 Nevertheless, there is only a limited investigation on the cyclic deformation behavior of the Ag nanowire/RGO hybrid electrode under bending strain whereupon only the ex situ resistance measurements were reported after imposing a small number of cycles.15,20–23 Furthermore, even though the surface oxidation is expected to degrade the mechanical properties of metal nanowires24 thereby resulting in poor reliability under bending fatigue, there is no study on the change in reliability after being exposed to an oxidation environment.
In the present work, the role of RGO layer in enhancing the chemical stability and its effect on the mechanical reliability were systematically studied for Ag nanowire/RGO hybrid transparent electrode. The RGO protective layer was deposited on top of the Ag nanowire network, and the mechanical reliability of the fabricated Ag nanowire/RGO hybrid electrodes were examined using a bending fatigue tester capable of in situ resistance measurements. Large number of cycles of up to 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles was imposed, and the resistance was monitored in situ during the bending fatigue tests. In addition, the effect of the surface oxidation of the Ag nanowires on the chemical stability as well as mechanical reliability were investigated by exposing samples to ambient air and then performing similar bending fatigue tests on the exposed Ag nanowire networks with and without RGO protective layer. Furthermore, we propose that further enhancement in the chemical stability and mechanical reliability can be achieved by replacing the thermal annealing with mechanical welding by applying simple bending strain to the Ag nanowire/RGO hybrid electrodes.
000 cycles was imposed, and the resistance was monitored in situ during the bending fatigue tests. In addition, the effect of the surface oxidation of the Ag nanowires on the chemical stability as well as mechanical reliability were investigated by exposing samples to ambient air and then performing similar bending fatigue tests on the exposed Ag nanowire networks with and without RGO protective layer. Furthermore, we propose that further enhancement in the chemical stability and mechanical reliability can be achieved by replacing the thermal annealing with mechanical welding by applying simple bending strain to the Ag nanowire/RGO hybrid electrodes.
2. Experimental
2.1. Fabrication of silver nanowire electrode
Here, thinner and longer Ag nanowires were synthesized by using the modified polyol process that is described in detail in the work by Kim et al.11 The synthesized Ag nanowire solution was sprayed onto flexible polyimide substrates (Dupont, Kepton E, 0.125 mm 4(W) × 70(L) × 0.125(T) mm) at room temperature by using an electrostatic spray system from Nano NC Inc. A thin polyvinylpyrrolidone (PVP) layer, which induces anisotropic growth,32 encapsulates the as-synthesized Ag nanowires, and the PVP layer act as an insulating layer, thereby causing high junction resistance. To decrease the resistance of Ag nanowire networks, therefore, a post-annealing process is typically needed,6 and here, a box furnace annealing was performed at 170 °C for 30 min. The sheet resistance of the fabricated Ag nanowire electrode was measured using a four point resistance measurement system (FPP-2400) from Dasol Eng Co., Ltd. In this study, samples with similar density of Ag nanowires were fabricated to study the effect of bending fatigue on the electrical property and the deformation behavior of the Ag nanowire network. The initial sheet resistance of the as-fabricated samples was 27.8–28.7 ohm sq−1, and the resistance reduced to 14.7–15.3 ohm sq−1 after annealing at 170 °C for 30 min. The total transmittance of the Ag nanowire network with initial sheet resistance of ∼15.3 ohm sq−1 is 91%.2.2. Synthesis of RGO solution and fabrication of Ag nanowire/RGO hybrid electrodes
Commercial GO suspension solution (graphene supermarket) was the starting material for RGO synthesis. Solvent was exchanged with pure DI water to remove residual ions in GO solution for purification. 5 μl of hydrazine solution (35 wt% in water, Aldrich) and 35 μl of ammonia solution (28 wt% in water, Aldrich) was added into 0.025 wt% of GO solution in 20 ml vial. Hydrazine is acted as reductant and ammonia is used for dispersion agent. The obtained solution was heated in a water bath at 95 °C for 1 h and in this step, color of this solution is changed from brown to black.To fabricate Ag nanowire/RGO hybrid electrodes, the RGO layers were formed on the fabricated Ag nanowire electrode on PI substrate by using the same electrostatic spray system at room temperature. The sheet resistance decreased with the amount of 0.1–0.3 ohm sq−1 after the RGO deposition with the volume of 45 ml on the annealed Ag nanowire networks. The amount of transmittance decrease was confirmed as ∼2% when RGO layers were coated on the Ag nanowire network. Since the RGO was air sprayed on already formed Ag nanowire network without further annealing steps, the RGO is physically put in contact with the underlying Ag nanowires rather than relying on any functional groups on RGO interacting with the underlying Ag nanowires.
2.3. Bending fatigue test
Cyclic bending fatigue tester was employed to perform fatigue tests on Ag nanowire networks on PI substrates. This bending tester is capable of high number of bending cycles up to more than 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under fixed, uniform strain conditions, while monitoring the resistance change in situ. Ag nanowire or Ag nanowire/RGO on PI specimen was mounted in between the two parallel plates using screw bolts, in which each plate has Cu pad that is in contact with the Ag nanowire network for resistance measurements during cyclic bending. To apply repeatable bending strain, the lower plate moves horizontally with a fixed plate motion distance that determines the strain induced area. A uniform strain is imposed by choosing the desired spacing between the two plates. According to ε = y/R, where y is the neutral plane which can be determined by the substrate thickness and R, the radius, means a half value of the distance between the plates, the nominal strain imposed on the nanowires for a given plate spacing is then calculated. The spacing between the plates, 2R, was fixed to 8.3 mm, which corresponds to nominal strain induced on the Ag nanowire networks of 1.5%. The number of bending cycles was chosen to be 800
000 cycles under fixed, uniform strain conditions, while monitoring the resistance change in situ. Ag nanowire or Ag nanowire/RGO on PI specimen was mounted in between the two parallel plates using screw bolts, in which each plate has Cu pad that is in contact with the Ag nanowire network for resistance measurements during cyclic bending. To apply repeatable bending strain, the lower plate moves horizontally with a fixed plate motion distance that determines the strain induced area. A uniform strain is imposed by choosing the desired spacing between the two plates. According to ε = y/R, where y is the neutral plane which can be determined by the substrate thickness and R, the radius, means a half value of the distance between the plates, the nominal strain imposed on the nanowires for a given plate spacing is then calculated. The spacing between the plates, 2R, was fixed to 8.3 mm, which corresponds to nominal strain induced on the Ag nanowire networks of 1.5%. The number of bending cycles was chosen to be 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and the tests were performed at the plate movement distances of 10 mm with the bending speed of 300 cycles per a minute. More detailed description of the cyclic bending fatigue tester can be found in ref. 12 and 33.
000 cycles, and the tests were performed at the plate movement distances of 10 mm with the bending speed of 300 cycles per a minute. More detailed description of the cyclic bending fatigue tester can be found in ref. 12 and 33.
3. Results and discussion
3.1. Characterization of RGO layers and Ag nanowire/RGO networks
RGO obtained from the hydrazine-reduction of graphene oxide (GO) that is suspended in methanol solution was electrostatically spray-coated on top of the Ag nanowire network to fabricate the RGO/Ag nanowire hybrid electrode. In order to first confirm that a uniform thin layer consisting of just a few-layers RGO sheets can be formed using spray deposition, RGO sprayed on oxidized Si wafer was characterized using atomic force microscopy (AFM) and X-ray photoelectron microscopy (XPS) [ESI 01†]. The line scan for the height profile using AFM showed that the average topographic height of RGO layers were ∼0.8 nm, which is consistent with the typical height of single layer graphene, 0.5–1.0 nm (Fig. S1a and b†).25–27 Chemical structure and composition of prepared RGO on oxidized Si wafer were then investigated by using XPS as shown in Fig. S1c and d.† The oxygen content of GO was 31.6% before reduction that decreased to 15.2% after reduction. In addition, C–C bonds dominant in RGO was clearly detected, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond that is characteristic of GO was hardly observed, thereby confirming that the RGO sheet prepared in this study was fully reduced to RGO.
O bond that is characteristic of GO was hardly observed, thereby confirming that the RGO sheet prepared in this study was fully reduced to RGO.
In order to confirm that the RGO layer uniformly covers the Ag nanowire network and therefore can function as charge transport layer in the transparent electrode, conductive AFM that is capable of measuring I–V curves with nanoscale scale lateral resolution was used [ESI 02†]. Fig. S2b† indicates the measured I–V curves of Ag nanowire/RGO hybrid electrode at two locations of first on top of the Ag nanowire and then in the area in between the nanowires, as marked in Fig. S2a.† Since the region in between the nanowires is covered with RGO, current flow was detected at both locations of on top as well as in between the Ag nanowires. Although the resistivity of RGO is relatively high (10−4 ohm m) in comparison to that of Ag nanowires (3 × 10−8 ohm m), the submicron travel distance to nearby Ag nanowires, however, allowed for sufficient current flow. Therefore, the capability of the RGO in providing alternative current path was demonstrated from this simple AFM analysis.
3.2. Bending fatigue behavior of Ag nanowire/RGO hybrid transparent electrodes
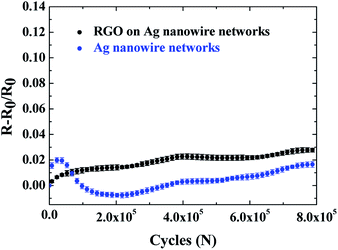
In order to examine how RGO deposition on top of the Ag nanowire network affects the deformation behavior of the Ag nanowire network, bending fatigue tests were performed on the Ag nanowire/RGO hybrid electrodes. The Ag nanowire transparent electrodes were prepared to have the initial sheet resistance of 14.7–15.3 ohm sq−1. Since the RGO layer is able to make additional current paths, the sheet resistance decreased by 0.1–0.3 ohm sq−1 after RGO deposition on the Ag nanowire networks. A uniform bending strain of 1.5% was imposed with the plate motion distance of 10 mm over 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The fractional resistance change ((R − R0)/R0) of Ag nanowire/RGO hybrid electrode over number of cycles is provided in Fig. 1. For comparison, a similar test was performed on bare Ag nanowire electrode without RGO protective layer, and the result is shown together in Fig. 1. Both the Ag nanowire and Ag nanowire/RGO hybrid electrodes have excellent mechanical reliability under bending fatigue that showed 1.6% and 2.7% increase in fractional resistance at the end of 800
000 cycles. The fractional resistance change ((R − R0)/R0) of Ag nanowire/RGO hybrid electrode over number of cycles is provided in Fig. 1. For comparison, a similar test was performed on bare Ag nanowire electrode without RGO protective layer, and the result is shown together in Fig. 1. Both the Ag nanowire and Ag nanowire/RGO hybrid electrodes have excellent mechanical reliability under bending fatigue that showed 1.6% and 2.7% increase in fractional resistance at the end of 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively. The increases in fractional resistance in both cases are significantly lower than in the case for Ag thin film, which showed ∼90% increase in fractional resistance under the same test conditions [ESI 03†]. Metal nanostructures display interesting size dependent plasticity,28,29,35 and the metal nanowires limited surface defects were reported to have high strengths reaching that of the theoretical strength of E/10.30,36,37 Such high strength of the individual nanowire is one reason for excellent reliability of the Ag nanowire network, in addition to the fact that the network geometry is well suited to accommodate stretching while reducing the strain on the individual nanowires.
000 cycles, respectively. The increases in fractional resistance in both cases are significantly lower than in the case for Ag thin film, which showed ∼90% increase in fractional resistance under the same test conditions [ESI 03†]. Metal nanostructures display interesting size dependent plasticity,28,29,35 and the metal nanowires limited surface defects were reported to have high strengths reaching that of the theoretical strength of E/10.30,36,37 Such high strength of the individual nanowire is one reason for excellent reliability of the Ag nanowire network, in addition to the fact that the network geometry is well suited to accommodate stretching while reducing the strain on the individual nanowires.
The change in fractional resistance in Ag nanowires with and without RGO during the bending fatigue tests observed in Fig. 1 is expected to be due to two modes of deformations. The first mode of failure involved deformation at the thermally locked-in junctions, which are formed during the thermal annealing process that is needed to remove PVP layers encapsulating the as-synthesized Ag nanowires from polyol reduction method.6,11 Since the thermally locked-in junctions are formed between two nanowires with different crystallographic orientations, grain boundaries must exist between the junction and the rest of the nanowire. Therefore, failure of the Ag nanowire networks under bending fatigue mainly occurs at thermally locked-in junctions, thereby increasing the resistance under fatigue. Although a thermal annealing step forms thermally locked-in junctions in Ag nanowire networks, there are still many Ag nanowires that do not form the locked-in junctions but rather lie on each other without contact formation for current path. Application of repeated bending strain to the stacked Ag nanowire networks can lead to the junction formations that are just enough to result in the needed electrical conductivity by mechanical welding effect as localized plasticity joins the nanowires at the contact instead of fusing the nanowires.12 The second mode of failure then involves progressive formation and subsequent failure of mechanically welded junctions. The failure of mechanically welded junctions can also cause an increase in the overall resistance under bending fatigue although mechanically welded junctions are reported to be more robust in reliability than the thermally locked-in junctions.12,38
Since both deformation mechanisms occur simultaneously during cyclic bending, however, the resistance change is dominated by which mechanism is more prominent at the cycle. In the case of the bare Ag nanowire networks of the present study, the failures occur fast at the weakest thermally locked-in junctions first, thereby, leading to the sharp increase in resistance in the initial stage of bending. Once the weakest links are eliminated from the network, however, the probability of junction formations by mechanically welding become more pronounced than the failures at the locked-in junctions. Therefore, a decrease in resistance was observed following the initially observed sharp increase in resistance. After the junction form via mechanical welding, an increase in resistance is observed at a slower rate than that in the initial stage that is indicative of further propagation of the fatigue failure near the remaining thermally locked-in junctions as well as mechanically welded junctions.
The fatigue behavior of the Ag nanowire/RGO hybrid electrodes can also be explained by the similar deformation mechanism where failure first occurs at the thermally locked-in junctions of Ag nanowire network. Fig. 2 shows a series of SEM images, which were taken for the Ag nanowire/RGO hybrid electrodes after imposing 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 100
000, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 800
000 and 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1.5% bending strain. It revealed that the failure of the Ag nanowire/RGO hybrid electrodes occurred at the thermally locked-in junctions, which resonates well with the failure mode of bare Ag nanowire network described above. Since the thin RGO layer just covers the nanowire networks without strong adhesive strength to the Ag nanowire network or the underlying substrate, the RGO deposition did not show a significant difference in the overall reliability. The RGO layers, even with full coverage of the substrate, allowed for the Ag nanowires to freely respond to the cyclic bending strains, thereby resulting in the similar deformation mechanism to the bare Ag nanowire network.
000 cycles at 1.5% bending strain. It revealed that the failure of the Ag nanowire/RGO hybrid electrodes occurred at the thermally locked-in junctions, which resonates well with the failure mode of bare Ag nanowire network described above. Since the thin RGO layer just covers the nanowire networks without strong adhesive strength to the Ag nanowire network or the underlying substrate, the RGO deposition did not show a significant difference in the overall reliability. The RGO layers, even with full coverage of the substrate, allowed for the Ag nanowires to freely respond to the cyclic bending strains, thereby resulting in the similar deformation mechanism to the bare Ag nanowire network.
One clear difference in the fatigue behavior between the bare Ag nanowire and the Ag nanowire/RGO hybrid transparent electrodes is that the decrease in fractional resistance shown in the bare Ag nanowire networks was not observed in the Ag nanowire/RGO hybrid transparent electrodes. The Ag nanowire/RGO hybrid electrode showed a continuous increase in fractional resistance without the decrease in resistance that was observed in the bare Ag nanowire network. Since the RGO deposition provides additional current path on top of the Ag nanowire networks before imposing a bending strain, the influence of the formation of mechanically welded junctions on the resistance decrease are negligible for the case of Ag nanowire/RGO hybrid electrodes. The current path formation by RGO was able to be confirmed by the 1–2% decrease in the sheet resistance of the Ag nanowire networks after RGO deposition, which was mentioned above.
3.3. Effects of RGO protective layer on chemical stability and mechanical reliability
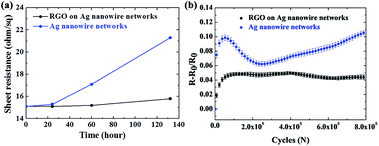
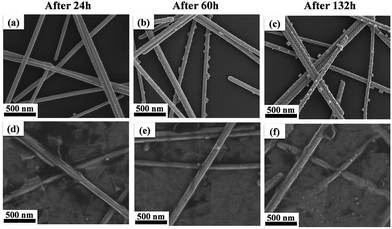
Surface oxidation of Ag nanowires occurs fast due to their large surface area to volume ratio when exposed to moist air, thereby resulting in resistance increase. However, deposition of RGO layer can prevent water or gas molecules in ambient air from coming in contact with the nanowires owing to its low permeability to gas or water molecules.13,16 In order to examine the enhancement of chemical stability by RGO deposition, bare Ag nanowire electrode and the Ag nanowire/RGO hybrid transparent electrodes were exposed to ambient air at 70 °C for 132 h. Fig. 3a presents the plot of sheet resistance change of the bare Ag nanowire and Ag nanowire/RGO hybrid transparent electrodes as a function of exposure time. While the bare Ag nanowire electrode showed an increase in sheet resistance from 15.1 to 21.3 ohm sq−1, a much smaller increase in sheet resistance from 15.1 to 15.8 ohm sq−1 was observed for the Ag nanowire/RGO hybrid transparent electrodes after 132 h of exposure. Since oxidation reduces the cross-section area of the metal samples that causes the deterioration of electrical conductance,31 the small increase in sheet resistance is indicative of less surface oxidation in the Ag nanowire/RGO hybrid electrode than in the bare Ag nanowire electrode.For more detailed investigation on the suppression of the surface oxidation by RGO deposition, SEM images were taken for the bare Ag nanowire and the Ag nanowire/RGO hybrid electrodes after being exposed to air at 70 °C for the different time. (Fig. 4a–c) As shown in Fig. 4a, small oxide particles covered the surface of the bare Ag nanowires after 24 h of exposure, and the number as well as the size of the oxide particles increased as the exposing time increased (Fig. 4b and c). The chemical composition of the surface particles were confirmed as silver oxide by EDX analysis (Fig. S4†), which must be Ag2O based on the chemical equilibrium.14 On the other hand, the surface oxidation was not evident in the Ag nanowire/RGO hybrid electrodes as shown in Fig. 4d and e. Only the specimen that was exposed for an extended period of 132 h showed some oxide particle formations on the nanowire surface (Fig. 4f and S4†). Thus, suppression of the oxidation is in agreement with smaller increase in sheet resistance when exposed to ambient air.
To understand the effect of surface oxidation on the mechanical reliability under bending fatigue, bending fatigue tests were performed on the bare Ag nanowire and Ag nanowire/RGO hybrid electrodes after exposing both to air at 70 °C for 132 h. The initial sheet resistance was ∼15.3 ohm sq−1 for both specimens that increased to ∼16.0 ohm sq−1 for the Ag nanowire/RGO hybrid electrode and ∼22.0 ohm sq−1 for the bare Ag nanowire electrode after the exposure, and the bending fatigue test results for both specimens are shown in Fig. 3b. At the end of the bending cycles, the Ag nanowire/RGO hybrid electrode showed significantly smaller increase in fractional resistance, 4.2%, compared to that of the bare Ag nanowire electrode, which had 10.9% increase. In addition, the bare Ag nanowire electrode showed continuous increase in fractional resistance even at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, while the Ag nanowire/RGO hybrid electrode sustained a steady-state without further increase in resistance until the end of the tests. If the fatigue progresses further to even larger number of cycles, the resistance increase is expected to be more pronounced in the bare Ag nanowire electrodes compared to that of the Ag nanowire/RGO hybrid electrodes.
000 cycles, while the Ag nanowire/RGO hybrid electrode sustained a steady-state without further increase in resistance until the end of the tests. If the fatigue progresses further to even larger number of cycles, the resistance increase is expected to be more pronounced in the bare Ag nanowire electrodes compared to that of the Ag nanowire/RGO hybrid electrodes.
Enhanced mechanical reliability of the Ag nanowire/RGO hybrid electrode after being exposed to air is attributed to the reduced surface oxidation on the Ag nanowires by the RGO layer. The failure of the Ag nanowire network is caused by the progression of plastic deformation near the locked-in junctions in response to the continuously applied bending strain.12 The brittle oxide particles on the nanowire surface, however, can act as stress concentrators near which the probability of possible crack/damage initiation increases, thereby leading to the deterioration of the mechanical properties of metal nanowires.24 Therefore, reducing the surface oxidation with RGO protective layer can minimize the mechanical properties degradation of Ag nanowires after being exposed to air that resulted in the decreased susceptibility to the fatigue failure of the Ag nanowire/RGO hybrid electrodes.
3.4. Further enhancement of mechanical reliability and chemical stability of Ag nanowire/RGO hybrid transparent electrode by mechanical welding
As mentioned above, the failure mechanism of the Ag nanowire/RGO hybrid electrodes was similar to that of the bare Ag nanowire electrodes, in which failure occurs at the thermally locked-in junctions. Therefore, the mechanical reliability of the Ag nanowire/RGO hybrid electrode can also be expected to be enhanced by decreasing the number of the locked-in junctions. In order to achieve that, we removed the thermal annealing step, which is the main cause for the formation of locked-in junctions, and used bending strain to the specimen to induce plasticity at the junction to form mechanically welded junctions (Fig. 5). The applied bending strain results in stress concentration at the junction of nanowires that results in localized plasticity, thus lowering the contact resistance.12 Detailed descriptions for the mechanically welded junction can be found in ref. 12. As-deposited bare Ag nanowire networks with sheet resistance of 28.5 ohm sq−1, decreased to 17.6 ohm sq−1 after RGO layer deposition since RGO provides additional current pathway.13,34 Next, bending strain was applied to the Ag nanowire/RGO hybrid electrode to form mechanically welded junctions. In situ fractional resistance change during cyclic bendings at 1.5% strain are shown in Fig. 5, and the sheet resistance decreased to 15.0 ohm sq−1 after 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and the end resistance is comparable to the value achievable by thermal annealing. No increase in fractional resistance was observed even at 800
000 cycles, and the end resistance is comparable to the value achievable by thermal annealing. No increase in fractional resistance was observed even at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles due to the absence of thermally locked-in junctions in the un-annealed Ag nanowire/RGO hybrid electrodes. Furthermore, the RGO deposition was successfully able to suppress the surface oxidation, which enhanced the chemical stability of the un-annealed Ag nanowire/RGO hybrid electrodes without an increase in sheet resistance even after exposure to air for 288 h at 70 °C. Therefore, further enhancement of mechanical reliability and chemical stability was demonstrated by using bending strain to form mechanically welded junctions for the Ag nanowire/RGO hybrid electrodes.
000 cycles due to the absence of thermally locked-in junctions in the un-annealed Ag nanowire/RGO hybrid electrodes. Furthermore, the RGO deposition was successfully able to suppress the surface oxidation, which enhanced the chemical stability of the un-annealed Ag nanowire/RGO hybrid electrodes without an increase in sheet resistance even after exposure to air for 288 h at 70 °C. Therefore, further enhancement of mechanical reliability and chemical stability was demonstrated by using bending strain to form mechanically welded junctions for the Ag nanowire/RGO hybrid electrodes.
4. Conclusion
In this study, the deformation behavior of the Ag nanowire/RGO hybrid transparent electrodes under bending fatigue was studied. Excellent reliability of the Ag nanowire networks was sustained even after the RGO deposition due to the high flexibility of the thin RGO layer. Furthermore, the low permeability of RGO to gas or water molecules resulted in higher chemical stability of the Ag nanowire/RGO hybrid electrode than that of the bare Ag nanowire electrode. SEM analysis confirmed that the surface oxidation, which is formed when exposed to humid air, was essentially prevented after RGO deposition. Since the surface oxidation degrades the mechanical properties of metal nanowires, suppression of the surface oxidation via RGO deposition was shown to enhance the reliability of the Ag nanowire/RGO hybrid electrode when exposed to oxidation environment during cyclic bendings. Lastly, the Ag nanowire/RGO hybrid electrodes, which were fabricated without a thermal annealing process after Ag nanowire deposition, showed enhanced mechanical reliability with the low sheet resistance.Acknowledgements
The authors would like to acknowledge the financial support from National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2016R1A2B3011473), Center for Advanced Meta-Materials funded by the Ministry of Science, ICT and Future Planning as Global Frontier Project (CAMM-No. 2014063701) and Technology Innovation Program and Industrial Strategic Technology Development Program (RCMS 10052790) under Korea Evaluation Institute of Industrial Technology (KEIT).Notes and references
- J. Lewis, S. Grego, B. Chalamala, E. Vick and D. Temple, Appl. Phys. Lett., 2004, 85(16), 3450 CrossRef CAS.
- M. W. Rowell, M. A. Topinka, M. D. McGehee, H.-J. r. Prall, G. Dennler, N. S. Sariciftci, L. Hu and G. Gruner, Appl. Phys. Lett., 2006, 88(23), 233506 CrossRef.
- D. Zhang, K. Ryu, X. Liu, E. Polikarpov, J. Ly, M. E. Tompson and C. Zhou, Nano Lett., 2006, 6(9), 1880–1886 CrossRef CAS PubMed.
- J. Wu, M. Agrawal, H. A. Becerril, Z. Bao, Z. Liu, Y. Chen and P. Peumans, ACS Nano, 2010, 4(1), 43–48 CrossRef CAS PubMed.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J. H. Ahn, P. Kim, J. Y. Choi and B. H. Hong, Nature, 2009, 457(7230), 706–710 CrossRef CAS PubMed.
- J.-Y. Lee, S. T. Connor, Y. Cui and P. Peumans, Nano Lett., 2008, 8(2), 689–692 CrossRef CAS PubMed.
- L. Hu, H. S. Kim, J.-Y. Lee, P. Peumans and Y. Cui, ACS Nano, 2010, 4(5), 2955–2963 CrossRef CAS PubMed.
- K. A. Sierros, N. J. Morris, K. Ramji and D. R. Cairns, Thin Solid Films, 2009, 517(8), 2590–2595 CrossRef CAS.
- Z. Chen, B. Cotterell and W. Wang, Eng. Fract. Mech., 2002, 69(5), 597–603 CrossRef.
- S. De, T. M. Higgins, P. E. Lyons, E. M. Doherty, P. N. Nirmalraj, W. J. Blau, J. J. Boland and J. N. Coleman, ACS Nano, 2009, 3(7), 1767–1774 CrossRef CAS PubMed.
- T. Kim, A. Canlier, G. H. Kim, J. Choi, M. Park and S. M. Han, ACS Appl. Mater. Interfaces, 2013, 5(3), 788–794 CAS.
- B. Hwang, H.-A. S. Shin, T. Kim, Y.-C. Joo and S. M. Han, Small, 2014, 10(16), 3397–3404 CrossRef CAS PubMed.
- Y. Ahn, Y. Jeong and Y. Lee, ACS Appl. Mater. Interfaces, 2012, 4(12), 6410–6414 CAS.
- Y.-J. Tsai, C.-Y. Chang, Y.-C. Lai, P.-C. Yu and H. Ahn, ACS Appl. Mater. Interfaces, 2014, 6(1), 630–635 CAS.
- D. Lee, H. Lee, Y. Ahn, Y. Jeong, D.-Y. Lee and Y. Lee, Nanoscale, 2013, 5(17), 7750–7755 RSC.
- H. W. Kim, H. W. Yoon, S. M. Yoon, B. M. Yoo, B. K. Ahn, Y. H. Cho, H. J. Shin, H. Yang, U. Paik, S. Kwon, J. Y. Choi and H. B. Park, Science, 2013, 342(6154), 91–95 CrossRef CAS PubMed.
- I. N. Kholmanov, M. D. Stoller, J. Edgeworth, W. H. Lee, H. Li, J. Lee, C. Barnhart, J. R. Potts, R. Piner, D. Akinwande, J. E. Barrick and R. S. Ruoff, ACS Nano, 2012, 6(6), 5157–5163 CrossRef CAS PubMed.
- K. Naito, N. Yoshinaga, E. Tsutsumi and Y. Akasaka, Synth. Met., 2013, 175, 42–46 CrossRef CAS.
- I. K. Moon, J. I. Kim, H. Lee, K. Hur, W. C. Kim and H. Lee, Sci. Rep., 2013, 3, 1112 Search PubMed.
- L. Yang, T. Zhang, H. Zhou, S. C. Price, B. J. Wiley and W. You, ACS Appl. Mater. Interfaces, 2011, 3(10), 4075–4084 CAS.
- C. H. Liu and X. Yu, Nanoscale Res. Lett., 2011, 6(1), 75 CrossRef PubMed.
- S. Wang, X. Zhang and W. Zhao, J. Nanomater., 2013, 2013, 1–6 Search PubMed.
- A. R. Madaria, A. Kumar and C. Zhou, Nanotechnology, 2011, 22(24), 245201 CrossRef PubMed.
- C. Peng, Y. Zhan and J. Lou, Small, 2012, 8(12), 1889–1894 CrossRef CAS PubMed.
- Y. Zhu, W. Cai, R. D. Piner, A. Velamakanni and R. S. Ruoff, Appl. Phys. Lett., 2009, 95(10), 103104 CrossRef.
- S. Park, J. An, I. Jung, R. D. Piner, S. J. An, X. Li, A. Velamakanni and R. S. Ruoff, Nano Lett., 2009, 9(4), 1593–1597 CrossRef CAS PubMed.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3(2), 101–105 CrossRef CAS PubMed.
- J. R. Greer, W. C. Oliver and W. D. Nix, Acta Mater., 2005, 53(6), 1821–1830 CrossRef CAS.
- J. R. Greer and W. D. Nix, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 24 CrossRef.
- J. H. Seo, Y. Yoo, N. Y. Park, S. W. Yoon, H. Lee, S. Han, S. W. Lee, T. Y. Seong, S. C. Lee, K. B. Lee, P. R. Cha, H. S. Park, B. Kim and J. P. Ahn, Nano Lett., 2011, 11(8), 3499–3502 CrossRef CAS PubMed.
- B. N. Roy and T. Wright, Cryst. Res. Technol., 1996, 31(8), 1039–1044 CrossRef CAS.
- Y. Sun, Y. Yin, B. T. Mayers, T. Herricks and Y. Xia, Chem. Mater., 2002, 14(11), 4736–4745 CrossRef CAS.
- B.-J. Kim, H.-A. S. Shin, S.-Y. Jung, Y. Cho, O. Kraft, I.-S. Choi and Y.-C. Joo, Acta Mater., 2013, 61(9), 3473–3481 CrossRef CAS.
- J. Liang, L. Li, K. Tong, Z. Ren, W. Hu, X. Niu, Y. Chen and Q. Pei, ACS Nano, 2014, 8(2), 1590–1600 CrossRef CAS PubMed.
- S. M. Han, G. Feng, J. Y. Jung, H. J. Jung, J. R. Groves, W. D. Nix and Y. Cui, Appl. Phys. Lett., 2013, 102, 041910 Search PubMed.
- B. Hwang, M. J. Kang, S. Lee, C. R. Weinberger, P. Loya, J. Lou, S.-H. Oh, B. Kim and S. M. Han, Nanoscale, 2015, 7, 15657–15664 RSC.
- B. Wu, A. Heidelberg and J. J. Boland, Nat. Mater., 2005, 4, 525–529 CrossRef CAS PubMed.
- B. Hwang, T. G. Kim and S. M. Han, Extreme Mechanics Letters, 2016 DOI:10.1016/j.eml.2016.02.011.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10338c |
| ‡ These authors contributed equally to the research. |
| This journal is © The Royal Society of Chemistry 2016 |