Fabrication of rutile TiO2 nanorod arrays on a copper substrate for high-performance lithium-ion batteries†
Guanrao Liu,
Shichao Zhang*,
Xiaomeng Wu and
Ruoxu Lin
School of Materials Science and Engineering, Beijing University of Aeronautics and Astronautics, Beijing 100191, P. R. China. E-mail: csc@buaa.edu.cn
First published on 26th May 2016
Abstract
A rutile TiO2 nanorod array has been successfully prepared on a flexible copper substrate and demonstrated as a high-performance anode material for lithium-ion batteries. The prepared materials exhibit excellent electrochemical performance, which depends crucially on the structural parameters and the large specific surface area of the array.
To achieve high areal energy and power densities within a limited area for next-generation lightweight Li-ion batteries (LIBs), tremendous effort has been devoted to developing new structured electrode materials.1–5 It is commonly recognized that titanium dioxide (TiO2) is a promising anode candidate for fast rechargeable LIBs. Li+ ion intercalation/de-intercalation in TiO2-based structures, including anatase, rutile, and TiO2–bronze (TiO2–B), are accompanied by negligible lattice changes, which can provide cells with good capacity retention on cycling, low self-discharge and enhanced safety, as well as environmental friendliness.6–9 Among them, rutile is the thermodynamically most stable structure of TiO2, and is also the most common natural form. However, bulk rutile can only accommodate <0.1 Li per TiO2 unit at room temperature,6,10 because of anisotropic Li+ diffusion in rutile structure. As shown in Fig. 1, in the ab-plane, there is almost no tunnel for Li+ transport due to the large lattice distortion, but there are channels parallel to the c-axis. Several impressive researches achieved to improve the Li+ intercalation properties of rutile TiO2 at room temperature, by reducing the particles dimension or solving the problem of irregular aggregation of particles.10–14
 | ||
| Fig. 1 Structural representation of rutile along the (a) [100], and (b) [001] directions. The octahedra are representative of TiO6. | ||
However, TiO2 electrodes based on powder materials still suffer from interfacial kinetic problems. The powder need to be mixed with a binder and conductive agent and further pressed onto current collecting substrates before they are suitable for integration into battery devices. This process introduces the additional risks of negating the benefits of electrochemistry using nanomaterials. In addition, efficient electron transport from the current collecting substrate to electrode materials cannot be ensured due to the possible presence of impurities at the interface. To solve these problems, it is an effective way of fabricating nanostructured arrays directly on the metallic current collecting substrates via in situ synthesis, by which the electrode materials possess better homogeneity, mechanical properties, as well as the excellent surface binding strength without any addictive.15–21 Such kind of arrays combines the advantages of both nanoscaled building blocks and the microstructures, i.e. the electrochemical performance of the electrode can be improved, owing to the high-active surface area, short path for ions, direct transport channel for electrons and specific structure stability. Almost all the reported rutile TiO2 arrays obtained in solutions were grown on ITO glass, Ti, or some carbon materials,22–28 which are not suitable for serving as current collector in practice because of the lack of flexibility, limited area or high-cost. Thus, their robust electrochemical applications for LIBs are restricted. In this work, self-supported rutile TiO2 arrays growing along c-axis fabricated directly on copper current collecting substrates via facile one-step in situ synthesis. The materials represent an attractive architecture as the electrode of LIBs, owing to the good contact of every nanostructure with the current collector, the high-efficiency of material utilization and the potential of large-area production.
The copper substrate (a thickness of 50 μm) for depositing TiO2 arrays was initially cleaned by sonicating in dilute sulfuric acid (0.5 M) and acetone, respectively. The arrays were prepared by hydrothermal treatment of 35 mL of TiCl3 (0.15 M) solution supersaturated with NaCl as well as 0.3 g of urea. The solution was placed in a Teflon-lined autoclave, and the as-cleaned copper substrate was placed in the solution standing against the wall of the autoclave. The autoclave was sealed and maintained at 160 °C for 2 h. The specimen was immersed in dilute sulfuric acid for 5 min and completely washed with distilled water and ethanol for several times. Finally, the sample was dried at 60 °C in vacuum oven for 10 h.
TiO2 arrays uniformly are grown on a 6 cm2 substrate. As is shown from Fig. 2a, the shining surface of the copper foil is covered by a piece of white film after hydrothermal treatment. The film can be easily rolled with the substrate, indicating the flexibility of the as-prepared film. Top-surface images of a typical as-synthesized nanorods array sample (Fig. 2b) clearly demonstrate a highly uniform and densely packed array of nanorods with tetragonal crystallographic planes. The inset image of Fig. 2b is the cross-sectional view of the same sample, clearly demonstrating the large-scale growth of aligned TiO2 nanorods with typical diameter and length of 100 nm and 800 nm, respectively. Selected area of Fig. 2b indicates that the nanorods grow radially from some spots and are self-assembled into micro- and nanoscale hierarchical structures like dandelion. TEM and HRTEM image (Fig. 2c and d) of a single rod indicates that the nanorods are well crystallized with clear lattice fringes parallel to the wall. The inter-plane distance is 0.323 nm, which can be indexed as (110) of rutile TiO2. According to the XRD pattern of the electrode sample, the peaks are attributed to tetragonal rutile-structured TiO2 (space group P42/mnm, JCPDS file no. 87-710) with the exception of the reflections from the copper substrate. Combined with the XRD data and TEM images, such features imply that the nanorods grow along the c-axis with four side surfaces enclosed by {110} planes with a preferred [001] orientation. Energy-dispersive X-ray spectrometry (EDS) analysis (ESI, Fig. S1†) confirms that the nanorods contain Ti and O without any chloride.
As is known, Ti(III) is not stable in aqueous solution, and easily to be hydrolyzed into TiOH2+, which can be oxidized to Ti(IV) by dissolved oxygen in the solution. The hydrolysis reaction of TiCl3 can be described as follows:22,28
| Ti3+ + H2O → TiOH2+ + H+ | (1) |
| TiOH2+ + O2 → Ti(IV) oxo species + O2− → TiO2 | (2) |
The Ti(IV) oxo species is assumed to be an intermediate between TiO2+ and TiO2, consisting of partly dehydrated polymeric Ti(IV) hydroxide.22–28 The anisotropic growth of 1D titania nanorods can be understood from shape-control chemistry.29–31 With the formation of a TiO2 nanocrystal under hydrothermal environment, Cl− ion can selectively adsorb onto the {110} plane, which suppress and accelerate further growth in the [110] and [001] direction, respectively. Urea can produce ammonia ligands and OH− by hydrolysis in the aqueous solution, which plays an important role here for the growth of nanorods on copper substrate (details in ESI, Fig. S2†): (1) slow down the hydrolysis of Ti3+ and afford simultaneously hydrolysis–condensation by olation of the Ti3+, which can control the nucleation progress.29,31–33 (2) Tailoring the pH value of the reaction solution preventing the corrosion of Cu foils by high concentration of Cl−,34,35 especially with the presence of H+.
The electrochemical performance of the TiO2 nanorods arrays on Cu substrate is studied by Swagelok-type two-electrode cells (details in ESI†). The Cu substrate, which does not alloy with metallic lithium, could function directly as current collectors and the rutile TiO2 arrays coating acted as the active material so that no other binding or conductive additives were introduced. Note that the accurate mass loading of the active material is difficult to measure because of partial corrosion of Cu substrate thus the capacity unit has to be selected as μA h cm−2.
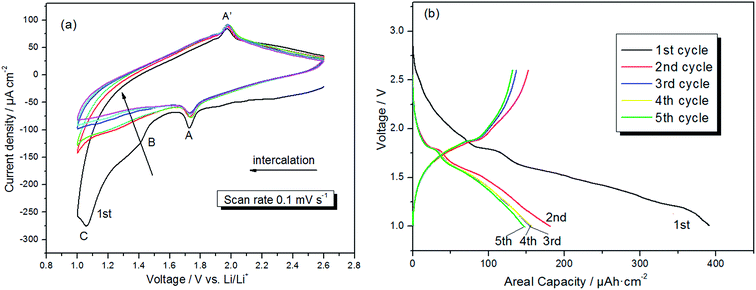
The cyclic voltammetry (CV) behavior of the prepared electrode in the range of 1 to 2.6 V (vs. Li/Li+), at a scan rate of 0.1 mV s−1 is depicted in Fig. 3a. In the initial cathodic sweep, several characteristic features occur. Li+ intercalation in single-crystal rutile structures manifests itself by a peak A at ∼1.73 V, a small broad peak B at ∼1.43 V, and an abrupt increase of cathodic current at potentials ∼1.1 V (peak C).36–40 Peak A corresponds to the conversion of TiO2 into LixTiO2 (TiO2 + xLi+ + xe− → LixTiO2), and peak A′ at ∼1.95 V represents the extraction of lithium ions from LixTiO2.36,37 Peak B and C disappears in the following scans, indicating that there are some irreversible reactions occurring. Solvent degradation is not a significant issue at potentials above 1.0 V, meaning that there is probably a phase transformation in the cycles.41 Therefore, the absence of peak B and C presumably attributed to the irreversible formation of the Li0.5TiO2 phase from the rutile crystals, which consumes some of the TiO2 and passivates the interfaces.38–40 Obviously, the 1D structures of rutile TiO2 grown along the c-axis enhanced the Li+ diffusion of electrode. In addition, the shapes of CV curves maintained after four cycles, indicating a good cycling performance. Fig. 3b displays the galvanostatic discharge/charge curves of the products cycled between 2.6–1.0 V at a rate of 5 μA cm−2. A total capacity of 386 μA h cm−2 is measured at the end of the first discharge, with a reversible capacity of 150 μA h cm−2. During the first discharge, the voltage plateau appeared at 1.73 V (vs. Li/Li+) corresponding to lithium intercalation into crystalline TiO2, and the charge potential plateau is observed at around 1.95 V, which are accordant with to the redox peaks in the CV.
The cycling performance of the prepared electrodes was further evaluated. At the current density of 5 μA cm−2 and 20 μA cm−2 (with a reversible specific discharge capacity of 123.6 μA h cm−2), the discharge capacity retentions were 40.4% and 48% from 1st to 2nd cycles respectively, and 38.8% and 37.6% over 50 cycles (Fig. 4a). The arrays exhibit an excellent high rate performance and efficiency when the current density increased to 100 to 200 μA cm−2. The reversible capacities of these current density were 91.2 μA h cm−2 and 75.6 μA h cm−2, with barely loss of the capacity after 100 cycles (Fig. 4b). The average columbic efficiency is above 95% at 100 μA cm−2 and higher rate. Fig. 4c depicts the morphologies of rutile TiO2 nanorods array cycled at current density of 200 μA cm−2 after 100 times. The array maintains organized 1D structure, as is known that TiO2 has no structural change during charge–discharge cycling. Compared to disorderly powder materials, the efficient electron transport from the current collector substrate to electrode materials and the existence of organized 1D morphology with [001] orientation contribute to a homogeneous insertion process.
Conclusions
A self-organized rutile TiO2 nanorods array is successfully assembled on flexible copper substrate by hydrothermal treatment without any template. The synthesis method is facile, low cost, and highly reproducible. The mechanism of 1D titania nanostructure growth can be understood from shape-control chemistry, by which the array was assembled by nanorods with an organized [001] orientation. The possibility of using this array as active electrode materials for lithium ion batteries was studied. For the metal substrate can be employed as current collector, no polymer binders and conductive agents were used. A specific reversible capacity up to 150 μA h cm−2 is achieved in lithium test cells for as-formed TiO2 nanorods that were 100 nm in diameter and 800 nm in length. The excellent electrochemical performance depends crucially on the structural properties of the arrays and the good contact of every nanostructure with the current collector, which presents an enormous potential application for LIBs with requirements of lightweight and flexibility.Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program) (2013CB934001), National Natural Science Foundation of China (51274017), National 863 Program of China (2013AA050904), International S & T Cooperation Program of China (2012DFR60530).Notes and references
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- D. Koziej, A. Lauria and M. Niederberger, Adv. Mater., 2014, 26, 235 CrossRef CAS PubMed.
- N. Liu, Z. Lu, J. Zhao, M. T. McDowell, H. W. Lee, W. Zhao and Y. Cui, Nat. Nanotechnol., 2014, 9, 187–192 CrossRef CAS PubMed.
- H. Wang and H. Dai, Chem. Soc. Rev., 2013, 42, 3088–3113 RSC.
- H. Li and H. Zhou, Chem. Commun., 2012, 48, 1201–1217 RSC.
- T. Ohzuku, Z. Takehara and S. Yoshizawa, Electrochim. Acta, 1978, 24, 219–222 CrossRef.
- A. R. Armstrong, G. Armstrong, J. Canales, R. García and P. G. Bruce, Adv. Mater., 2005, 17, 862–865 CrossRef CAS.
- G. F. Ortiz, I. Hanzu, T. Djenizian, P. Lavela, J. L. Tirado and P. Knauth, Chem. Mater., 2009, 21, 63–67 CrossRef CAS.
- X. Y. Yu, H. B. Wu, L. Yu, F. X. Ma and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 4001–4004 CrossRef CAS PubMed.
- L. Kavan, D. Fattakhova and P. Krtil, J. Electrochem. Soc., 1999, 146, 1375–1379 CrossRef CAS.
- Y. S. Hu, L. Kienle, Y. G. Guo and J. Maier, Adv. Mater., 2006, 18, 1421–1426 CrossRef CAS.
- N. A. Milne, M. Skyllas-Kazacos and V. Luca, J. Phys. Chem. C, 2009, 113, 12983–12995 CAS.
- J. S. Chen and X. W. Lou, J. Power Sources, 2010, 195, 2905–2908 CrossRef CAS.
- X. Li, C. M. Zhang and T. Meng, RSC Adv., 2016, 6, 4321–4328 RSC.
- L. Kavan, M. Kalbac, M. Zukalova, I. Exnar, V. Lorenzen, R. Nesper and M. Graetzel, Chem. Mater., 2004, 16, 477–485 CrossRef CAS.
- J. P. Liu, Y. Y. Li, X. T. Huang, R. M. Ding, Y. Y. Hu, J. Jiang and L. Liao, J. Mater. Chem., 2009, 19, 1859–1864 RSC.
- Y. S. Jung, A. S. Cavanagh, L. A. Riley, S. H. Kang, A. C. Dillon, M. D. Groner, S. M. George and S. H. Lee, Adv. Mater., 2010, 22, 2172–2176 CrossRef CAS PubMed.
- T. Djenizian, I. Hanzu and P. Knauth, J. Mater. Chem., 2011, 21, 9925–9937 RSC.
- B. Han, S. C. Zhang, R. Zhou, X. M. Wu, X. Wei, Y. L Xing, S. B. Wang and T. Qi, RSC Adv., 2014, 4, 50752–50758 RSC.
- K. Cheng, F. Yang, K. Ye, Y. Zhang, X. Jiang, J. L. Yin, G. L. Wang and D. X. Cao, J. Power Sources, 2014, 258, 260–265 CrossRef CAS.
- Q. Li, X. G. Miao, C. X. Wang and L. W. Yin, J. Mater. Chem. A, 2015, 3, 21328–21336 CAS.
- E. Hosono, S. Fujihara, K. Kakiuchi and H. Imai, J. Am. Chem. Soc., 2004, 126, 7790–7791 CrossRef CAS PubMed.
- X. G. Peng, L. Manna, W. D. Yang, E. S. Wickham, A. Kadavanich and A. P. Alivisatos, Nature, 2000, 404, 59–61 CrossRef CAS PubMed.
- A. S. Barnard and L. A. Curtiss, Nano Lett., 2005, 5, 1261–1266 CrossRef CAS PubMed.
- X. J. Feng, K. Shankar, O. K. Varghese, M. Paulose, T. J. Latempa and C. A. Grimes, Nano Lett., 2008, 8, 3781–3785 CrossRef CAS PubMed.
- S. M. Dong, H. B. Wang, L. Gu, X. H. Zhou, Z. H. Liu, P. X. Han, Y. Wang, X. Chen, G. L. Cui and L. Q. Chen, Thin Solid Films, 2011, 519, 5978–5982 CrossRef CAS.
- L. F. He, R. G. Ma, N. Du, J. G. Ren, T. L. Wong, Y. Y. Li and S. T. Lee, J. Mater. Chem., 2012, 22, 19061–19066 RSC.
- W. X. Guo, C. Xu, X. Wang, S. H. Wang, C. F. Pan, C. J. Lin and Z. L. Wang, J. Am. Chem. Soc., 2012, 134, 4437–4441 CrossRef CAS PubMed.
- L. Vayssieres and M. Graetzel, Angew. Chem., Int. Ed., 2004, 43, 3666–3670 CrossRef CAS PubMed.
- H. Wang, Z. Chen, Y. Leung, C. Luan, C. Liu, Y. Tang, C. Yan, W. Zhang, J. A. Zapien, I. Bello and S. Lee, Appl. Phys. Lett., 2010, 96, 263104–2603106 CrossRef.
- Y. B. Zhang, X. J. Feng and L. Jiang, Sci. China, Ser. B: Chem., 2007, 50, 175–178 CrossRef CAS.
- Y. H. Jin, S. H. Lee, H. W. Shim, K. H. Ko and D. W. Kim, Electrochim. Acta, 2010, 55, 7315–7321 CrossRef CAS.
- K. C. Song and Y. Kang, Mater. Lett., 2000, 42, 283–289 CrossRef CAS.
- J. L. Lei, L. J. Li, S. M. Cai, S. T. Zhang, D. Li and M. Z. Yang, Acta Phys.–Chim. Sin., 2001, 17, 1101–1111 Search PubMed.
- L. Nunez, E. Reguera, F. Corvo, E. Gonzalez and C. Vazquez, Corros. Sci., 2005, 47, 461–484 CrossRef CAS.
- D. H. Wang, D. W. Choi, Z. G. Yang, V. V. Viswanathan, Z. M. Nie, C. M. Wang, Y. J. Song, J. G. Zhang and J. Liu, Chem. Mater., 2008, 20, 3435–3442 CrossRef CAS.
- J. S. Chen, Y. L. Tan, C. M. Li, Y. L. Cheah, D. Y. Luan, S. Madhavi, F. Y. C. Boey, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 6124–6130 CrossRef CAS PubMed.
- Z. Hong, M. Wei, T. Lan, L. Jiang and G. Cao, Energy Environ. Sci., 2012, 5, 5408–5413 CAS.
- K. Siwinska-Stefanska and B. Kurc, J. Power Sources, 2015, 299, 286–292 CrossRef CAS.
- M. Marinaro, M. Pfanzelt, P. Kubiak, R. Marassi and M. Wohlfahrt-Mehrens, J. Power Sources, 2011, 196, 9825–9829 CrossRef CAS.
- M. V. Koudriachova, N. M. Harrison and S. W. Leeuw, Phys. Rev. Lett., 2001, 86, 1275–1278 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10285a |
| This journal is © The Royal Society of Chemistry 2016 |