High-performance flexible electron field emitters fabricated from doped crystalline Si pillar films on polymer substrates†
Abstract
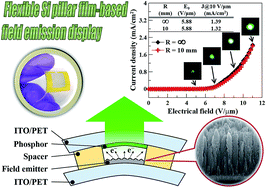
We report a new approach for the synthesis of various crystalline Si nanostructures on a polyimide (PI) substrate via microwave plasma enhanced chemical vapor deposition (MWPECVD) using SiCl4/H2 as precursors, and study the effects of conducting type (i.e., intrinsic, n-type, and p-type) on the electron field emission (EFE) properties of the Si nanostructures. H2 plasma treated B-doped crystalline Si pillars (H2: p-Si pillars) with a diameter of 50 nm and a sharp tip radius of 16 nm on a Mo-coated PI substrate reveals the best EFE performance with a low turn-on field of 5.85 V μm−1, high current density of 1.37 mA cm−2@10 V μm−1, and an extremely high field enhancement factor of 1281.13. This superior EFE performance is achieved because of its geometric features and high conductivity across the emitters. In addition, a flexible crystalline Si film-based field emission prototype device using the H2: p-Si pillar sample as the cathode is constructed. No obvious deterioration on EFE characteristics is observed when the device is subjected to bending at a radius of curvature (R) of 10 mm. According to the lifetime test, we achieve a half-life time over 10 h when a repeating FE-on/off test of 9 times at an R of 10 mm is performed, indicating high flexibility and good stability. These results thus demonstrate important steps toward a low-cost approach for creating high-performance and flexible field emission displays.


 Please wait while we load your content...
Please wait while we load your content...