Prochiral alkyl-aminomethyl ketones as convenient precursors for efficient synthesis of chiral (2,3,5)-tri-substituted pyrrolidines via an organo-catalysed tandem reaction†
Yuming Song*,
Ke Li,
Tao Shu,
Yamei Xie,
Yixin Zhang and
Jingping Qu
State Key Laboratory of Fine Chemicals, School of Pharmaceutical Science and Technology, Dalian University of Technology, Dalian 116024, P. R. China. E-mail: song-ym@dlut.edu.cn
First published on 31st May 2016
Abstract
α-Aminomethyl alkyl ketones reacted smoothly with α,β-unsaturated aldehydes via a tandem process catalysed by diphenylprolinol silyl ether/4-bromobenzoic acid. 2-Alkanoyl-3-aryl-5-hydroxy pyrrolidines were prepared in high yields (up to 99%) and enatioselectivities (>99% ee). The absolute configurations of the products were unambiguously confirmed by single-crystal diffraction.
α-Aminoketones and the masked versions are indispensable building blocks for the synthesis of natural products, pharmaceuticals and related intermediates.1–4 Asymmetric functionalization of prochiral α-aminoketones to create novel chiral scaffolds proved to be one of the most attractive approaches in organic synthesis. However, these research studies were greatly retarded because of the sensitivities of amino or keto groups or the relatively inert reactivity of α-C to common asymmetric transformations. In contrast, α-nitro5–9 or azido10–13 ketones were always used as the substituents for asymmetric aldol, Michael addition or tandem reactions to construct various complex chiral molecules.
Continuous efforts have also been made for the enantioselective C–C bond formation of α-aminoketones and promising results were obtained with substituted indolin-3-ones in asymmetric alkylation, allylation, Michael addition and some cascade reactions.14–20 The indolinone scaffold was assumed to dramatically improve the activities of α-C. In 2013, Vicario et al. reported the first asymmetric Michael/hemiaminal formation cascade process of acyclic aminoacetophenones with α,β-unsaturated aldehydes following an iminium activation mechanism catalysed by a di-(3,5-di-CF3)-phenyl-prolinol silyl ether/DABCO system. The phenyl, Ts groups combined with DABCO were assumed to facilitate the formation of active anion intermediate. At the same time, a complex mixture was obtained because of the lability of 5-OH group together with unsatisfied 2,3-cis/trans selectivity. Following an elegant procedure involving oxidation, isomerization, deprotection and ring opening, the products could be easily transformed to chiral γ-amino-δ-keto esters in excellent ees.21 Compared with their cyclic or acyclic aryl substituted counterparts, alkyl aminomethyl ketones were not extensively explored in asymmetric catalysis because of relatively lower activity. In 2008, Alexandre Alexakis et al. reported that alkyl aminomethyl ketones were excellent nucleophiles in asymmetric Michael addition with nitroolefins following an enamine catalysed mechanism.22 However, using α,β-unsaturated aldehydes as the electrophiles in similar transformations gave unsatisfied results. Vicario group explored preliminarily the reaction of amino acetone with (E)-crotonaldehyde, and chiral pyrrolidine was produced in moderate yield and ee (53%, 70% ee) via an aza-Michael/intramolecular aldol reaction cascade.21 To construct the key chiral polyfunctionalized cyclohexanone intermediate en route to (+)-trans-dihydrolycoricidine, McNulty group isolated an O bridged bis-pyrrolidine product in 14% yield from the reaction of N-Ts amino acetone and cinnamaldehyde with a secondary amine catalysis.11 Besides the α-C, proton containing α′ position in alkyl aminomethyl ketones is also active which greatly complicated the reactions.23
As our continuous efforts to prepare novel chiral heterocycles for medicinal uses, special attentions were paid to highly efficient reaction of alkyl aminomethyl ketone with α,β-unsaturated aldehyde for the preparation of chiral 2-alkanoyl-3-aryl pyrrolidines, which or their derivatives are widely used as monoamine reuptake inhibitors, the lifespan of eukaryotic organisms reagents, neuraminidase inhibitors and some other antagonists or antiviral agents (Fig. 1).24 And the additional hydrogen containing α′ position next to carbonyl group together with a stable 5-OH group, which may experience stereo specific intra- or inter-molecular transformations, are essential during the synthesis of some natural products and pharmaceuticals. Similar stable OH groups were reported in some chiral pyrrolidines.24–26
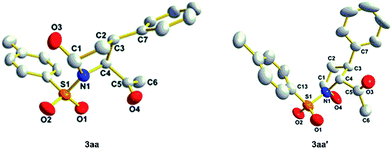
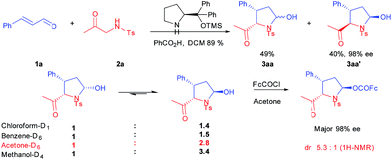
We initiated our investigation with a traditional diphenylprolinol silyl ether/benzoic acid/CH2Cl2 system using Ts-amino acetone (2a) and cinnamaldehyde (1a) as the substrates. To our delight, a mixture of 2,3-cis (3aa) and 2,3-trans (3aa′) pyrrolidines was obtained in excellent total yield (89%). However, from the proton NMR spectrum, the major 2,3-cis product contains 5-hydroxyl isomers, which could not be separated by HPLC. On the contrary, pure 2,3-trans isomer was obtained in 40% yield and up to 98% ee. Crystal structures of both 3aa and 3aa′ were obtained (PE/EA), and the 2,3-cis isomer retains a (2S,3S,5R) configuration while that of the 2,3-trans isomer adopts a (2R,3S,5S) configuration (Fig. 2). Encouraged by the promising results, the stability of 5-OH group in 3aa was carefully studied with 1H NMR in different solvents. We found that the hydroxyl group tautomerized soon and the isomer ratio varied with solvent change. Moreover, this isomerization was completely ceased in its ferrocene carboxylate derivative. A dynamic resolution process was observed during the esterification and the major ester product was isolated in 98% ee, that the mole ratio of esters was greatly improved compared with original isomers (from 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5.3
1 to 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Scheme 1). Compared with Vicario's procedure affording satisfying results with a di-(3,5-di-CF3)-phenylprolinol silyl ether/base system, current transformation with simple diphenylprolinol TMS ether under acidic conditions could further expand the substrates scope for α-aminomethyl alkyl ketones and some base sensitive substrates.
1) (Scheme 1). Compared with Vicario's procedure affording satisfying results with a di-(3,5-di-CF3)-phenylprolinol silyl ether/base system, current transformation with simple diphenylprolinol TMS ether under acidic conditions could further expand the substrates scope for α-aminomethyl alkyl ketones and some base sensitive substrates.
The reaction was further optimized with 3-(4-CN)-phenyl acraldehyde (1b) as the electrophile and the results were listed in Table 1. The activities and selectivities of the reactions varied with the protective group used. Ts proved to be the most active protection group; up to 91% of the products were isolated and excellent enantioselectivities were obtained for both isomers (>99% ees). Ms amino acetone 2b gave the best 2,3-cis selectivity (cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans = 3.3
trans = 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 62% yield but inferior enantioselectivity for the major cis-isomer (92% ee, ferrocene carboxylate). Tf protection can markedly increase the activity of the reaction under basic condition through an anion intermediate,21 however, 2c was completely inactive under current reaction conditions. We ascribe this to the neutral enol active intermediate formed during the reaction under weakly acidic conditions (Table 1 entry 1–3). Bulky alkyl substituted aminoketone 2, although less active, remarkably increased the 2,3-cis selectivities. When 2a, 2d or 2e were employed, 2,3-cis/2,3-trans ratio increased from 1.3
1) in 62% yield but inferior enantioselectivity for the major cis-isomer (92% ee, ferrocene carboxylate). Tf protection can markedly increase the activity of the reaction under basic condition through an anion intermediate,21 however, 2c was completely inactive under current reaction conditions. We ascribe this to the neutral enol active intermediate formed during the reaction under weakly acidic conditions (Table 1 entry 1–3). Bulky alkyl substituted aminoketone 2, although less active, remarkably increased the 2,3-cis selectivities. When 2a, 2d or 2e were employed, 2,3-cis/2,3-trans ratio increased from 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3.3
1 to 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with excellent ees (>95% ee). When 2f was employed, no 2,3-trans isomer was observed, stable 5-OH isomers (dr: 3.3
1 with excellent ees (>95% ee). When 2f was employed, no 2,3-trans isomer was observed, stable 5-OH isomers (dr: 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were obtained in good activities (53%), and excellent ee (>99%). α-Substituted substrate such as 2-aminomethyl cyclohexanone 2g was non-active for current reaction (Table 1 entries 3–7). Improved yield and identical selectivities were obtained under prolonged reaction time (from 20 h to 48 h) (Table 1 entry 8). Co-catalyst 4-halogen benzoic acid were evaluated, all the reactions showed excellent enantioselectivities (>98% ee) and with 4-bromobenzoic acid furnishing the 2,3-cis isomer in good yield and excellent dr (61%, dr 15
1) were obtained in good activities (53%), and excellent ee (>99%). α-Substituted substrate such as 2-aminomethyl cyclohexanone 2g was non-active for current reaction (Table 1 entries 3–7). Improved yield and identical selectivities were obtained under prolonged reaction time (from 20 h to 48 h) (Table 1 entry 8). Co-catalyst 4-halogen benzoic acid were evaluated, all the reactions showed excellent enantioselectivities (>98% ee) and with 4-bromobenzoic acid furnishing the 2,3-cis isomer in good yield and excellent dr (61%, dr 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 1 entries 8–11). The reaction media were evaluated, and the reaction in DCM give the desired product with the best dr (15
1) (Table 1 entries 8–11). The reaction media were evaluated, and the reaction in DCM give the desired product with the best dr (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and up to 98% ee (Table 1 entries 11–16).
1) and up to 98% ee (Table 1 entries 11–16).
| Entry | 2 | R | Acid | Solvent | Product | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|---|---|
| a Reactions performed with 1b (0.2 mmol) and 2a–g (0.25 mmol).b Isolated yield.c Determined by 1H NMR of the crude mixture.d Determined by HPLC on chiral stationary phase.e Reaction time is 20 h.f 2,3-cis/2,3-trans.g ee of ferrocene carboxylate (Fc).h Ratio of 5-hydroxy isomers.i Reaction time is 48 h. | ||||||||
| 1 | 2a | Ts | BA | CH2Cl2 | 3ba | 91e | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1f 1f |
>99g/>99 |
| 2 | 2b | Ms | BA | CH2Cl2 | 3bb | 62e | 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1f 1f |
92g/99g |
| 3 | 2c | Tf | BA | CH2Cl2 | 3bc | n.d. | n.d. | n.d. |
| 4 | 2d | Ts | BA | CH2Cl2 | 3bd | 68e | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1f 1f |
96g/97 |
| 5 | 2e | Ts | BA | CH2Cl2 | 3be | 61e | 2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1f 1f |
98g/97 |
| 6 | 2f | Ts | BA | CH2Cl2 | 3bf | 53e | 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
>99 |
| 7 | 2g | Ts | BA | CH2Cl2 | 3bg | n.d. | n.d. | n.d. |
| 8 | 2f | Ts | BA | CH2Cl2 | 3bf | 96d | 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
>99 |
| 9 | 2f | Ts | 4-F-BA | CH2Cl2 | 3bf | 82i | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
98 |
| 10 | 2f | Ts | 4-Cl-BA | CH2Cl2 | 3bf | 55i | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
99 |
| 11 | 2f | Ts | 4-Br-BA | CH2Cl2 | 3bf | 61i | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
98 |
| 12 | 2f | Ts | 4-I-BA | CH2Cl2 | 3bf | 52i | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
98 |
| 13 | 2f | Ts | 4-Br-BA | Toluene | 3bf | 62i | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
99 |
| 14 | 2f | Ts | 4-Br-BA | CH3OH | 3bf | 36i | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
98 |
| 15 | 2f | Ts | 4-Br-BA | THF | 3bf | 64i | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
98 |
| 16 | 2f | Ts | 4-Br-BA | EtOAc | 3bf | 63i | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1h 1h |
99 |
The scope of α,β-unsaturated aldehydes 1 was examined under the optimized conditions with increased catalysts loading and prolonged reaction time for better yields, (1 1.00 eq.; 2 1.25 eq.; Cat. 20 mol%; 4-Br Bz 20 mol%; in DCM 0.2 M; rt, 96 h). All reactions proceeded well, and the corresponding (2,3,5)-tri substituted chiral pyrrolidines were obtained in excellent enantioselectivities ranging from 87% to >99% ees (Table 2). With 2f as the nucleophile, neither electron-withdrawing nor electron-donating substituents on the phenyl unit of the acraldehydes appeared to significantly affect the enantioselectivities of the major products (>97% ee). On the other hand, those substitutions were closely related to their activities and diastereoselectivities, namely, electron deficient substrates proved to be more active and diastereoselective than their electron rich counterparts (Table 2 entry 1–4, 6, 7). With 1b or 1c as the substrate, the products were obtained with the best diastereoselectivity (dr 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), up to 81% yield and >99% ee, while that of with 4-methoxylphenyl substitution delivered product with 8
1), up to 81% yield and >99% ee, while that of with 4-methoxylphenyl substitution delivered product with 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 41% yield and 99% ee (Table 2 entries, 2, 3, 6). The substitution position also plays essential role for the reaction selectivity, compared with 1b, 1e has identical activity, enantioselectivity but much lower diastereoselectivity (4
1 dr, 41% yield and 99% ee (Table 2 entries, 2, 3, 6). The substitution position also plays essential role for the reaction selectivity, compared with 1b, 1e has identical activity, enantioselectivity but much lower diastereoselectivity (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2 entry 5). With electron rich 3-(2-furanyl) or 3-(1-naphthyl) acraldehyde as the substrates, relatively lower yields and enantioselectivities were obtained (Table 2 entry 8, 9). Besides stereo-hindered aminomethyl ketones, reactions of 3-(9-anthranyl) acraldehyde 1j with less hindered aminomethyl ketones furnished the chiral pyrrolidines in excellent activities, diastereoselectivities and enantioselectivities. With amino acetone 2a, the reaction provided predominately 2,3-cis isomer in 96% yield, excellent dr (25
1) (Table 2 entry 5). With electron rich 3-(2-furanyl) or 3-(1-naphthyl) acraldehyde as the substrates, relatively lower yields and enantioselectivities were obtained (Table 2 entry 8, 9). Besides stereo-hindered aminomethyl ketones, reactions of 3-(9-anthranyl) acraldehyde 1j with less hindered aminomethyl ketones furnished the chiral pyrrolidines in excellent activities, diastereoselectivities and enantioselectivities. With amino acetone 2a, the reaction provided predominately 2,3-cis isomer in 96% yield, excellent dr (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and >99% ee (Table 2 entry 10–12). We envisioned that a bigger repulsion between the anthranyl and alkanoyl should be responsible for the high levels of selectivity. Aminoacetone reacted with substituted cinnamaldehydes giving both 2,3-cis and 2,3-trans isomers in excellent activities, enantioselectivities but poor diastereoselectivities (Table 2 entry 13–15).
1) and >99% ee (Table 2 entry 10–12). We envisioned that a bigger repulsion between the anthranyl and alkanoyl should be responsible for the high levels of selectivity. Aminoacetone reacted with substituted cinnamaldehydes giving both 2,3-cis and 2,3-trans isomers in excellent activities, enantioselectivities but poor diastereoselectivities (Table 2 entry 13–15).
| Entry | 1 (R=) | 2 (Alk=) | 3 | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|
| a Reactions were performed with 1a–j (0.4 mmol) and 2c–f (0.5 mmol).b Isolated yield.c Determined by 1H NMR of the crude mixture (5-OH isomers).d Determined by HPLC on chiral stationary phase.e ee of ferrocene carboxylate was determined.f Determined by 1H NMR of the crude mixture. (A featured methyl of 2,3-cis isomer is right in the shielding zone of the adjacent aryl group. 5-OH dr ratio was not determined.) | ||||||
| 1 | 1a (C6H5-) | 2f (t-Bu) | 3af | 75 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
| 2 | 1b (4-CNC6H4-) | 2f (t-Bu) | 3bf | 79 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 3 | 1c (4-NO2C6H4-) | 2f (t-Bu) | 3cf | 81 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99 |
| 4 | 1d (4-BrC6H4-) | 2f (t-Bu) | 3df | 64 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
| 5 | 1e (3-NO2C6H4-) | 2f (t-Bu) | 3ef | 84 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 |
| 6 | 1f (4-OCH3C6H4-) | 2f (t-Bu) | 3ff | 41 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99e |
| 7 | 1g (3,4,5-OCH3C6H4-) | 2f (t-Bu) | 3gf | 70 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99e |
| 8 | 1h (R = 2-furanyl) | 2f (t-Bu) | 3hf | 43 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 9 | 1i (1-naphthyl) | 2f (t-Bu) | 3if | 44 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 10 | 1j (9-anthranyl) | 2a (Me) | 3ja | 96 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99 |
| 11 | 1j (9-anthranyl) | 2d (et) | 3jd | 96 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 |
| 12 | 1j (9-anthranyl) | 2e (i-Pr) | 3je | 92 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 |
| 13 | 1c (4-NO2C6H4-) | 2a (Me) | 3ca/3ca′ | 98 | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1f 1f |
>99e/>99e |
| 14 | 1d (4-BrC6H4-) | 2a (Me) | 3da/3da′ | 90 | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1f 1f |
97e/99e |
| 15 | 1f (4-OCH3C6H4-) | 2a (Me) | 3fa/3fa′ | 99 | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1f 1f |
>99e/97e |
A plausible mechanism for the sequential catalysis is depicted in (Scheme 2). Upon condensation with the secondary amine catalyst, an iminium intermediate, which is attacked by a nucleophile from less stereo-hindered lower side, is formed and pyrrolidine cycle is formed following a relay process. The stereochemistry of the product depends on the following factors: (a) the nitrogen adopts an approximate planar conformation and the sp3 hybrid Ts group should keep at the far-end of the iminium part; (b) in the stable conformation of the nucleophiles, α-H should be located at the same side with Ts group; (c) when 1j was employed, the stable iminium intermediate dramatically inhibits the 2,3-trans isomer formation because of the shielding effect of anthranyl group.
In conclusion, prochiral alkyl aminomethyl ketones successfully reacted with 3-aryl acraldehydes following a Michael/hemiaminal formation tandem procedure catalysed by diarylprolinol silyl ether/4-bromo benzoic acid and chiral poly functionalized pyrrolidines were obtained in up to 99% yield, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and >99% ee. With stereo-hindered substrates such as 2e or 1j, only 2,3-cis isomers were obtained generally in excellent ees, drs and yields. The absolute stereochemistry of both 2,3-cis and 2,3-trans isomers were characterized with X-ray single crystal diffraction. Preparing novel scaffolds based on the current reaction following stereo specific intra or inter molecular transformations is in progress.
1 dr and >99% ee. With stereo-hindered substrates such as 2e or 1j, only 2,3-cis isomers were obtained generally in excellent ees, drs and yields. The absolute stereochemistry of both 2,3-cis and 2,3-trans isomers were characterized with X-ray single crystal diffraction. Preparing novel scaffolds based on the current reaction following stereo specific intra or inter molecular transformations is in progress.
Acknowledgements
We thank the Fundamental Research Funds for the Central Universities (No. DUT14LAB19) for financial support. We also thank Prof. Baomin Wang, Prof. Yuhan Zhou, and Prof. Ying Peng for valuable discussions.Notes and references
- I. Moiseev, N. Makarova and M. Zemtsova, Chem. Heterocycl. Compd., 1999, 35, 637–649 CrossRef CAS.
- F. D. Klingler, Acc. Chem. Res., 2007, 40, 1367–1376 CrossRef CAS PubMed.
- J. M. Concellon and H. Rodriguez-Solla, Curr. Org. Chem., 2008, 12, 524–543 CrossRef CAS.
- S. Baktharaman, R. Hili and A. K. Yudin, Aldrichimica Acta, 2010, 41, 11 Search PubMed.
- R. Ballini, G. Bosica, D. Fiorini and A. Palmieri, Tetrahedron, 2005, 61, 8971–8993 CrossRef CAS.
- B. R. Linton, M. H. Reutershan, C. M. Aderman, E. A. Richardson, K. R. Brownell, C. W. Ashley, C. A. Evans and S. J. Miller, Tetrahedron Lett., 2007, 48, 1993–1997 CrossRef CAS.
- M. Jörres, I. Schiffers, I. Atodiresei and C. Bolm, Org. Lett., 2012, 14, 4518–4521 CrossRef PubMed.
- M. Jörres, S. Mersmann, G. Raabe and C. Bolm, Green Chem., 2013, 15, 612–616 RSC.
- Y.-n. Xuan, Z.-y. Chen and M. Yan, Chem. Commun., 2014, 50, 10471–10473 RSC.
- N. S. Chowdari, M. Ahmad, K. Albertshofer, F. Tanaka and C. F. Barbas, Org. Lett., 2006, 8, 2839–2842 CrossRef CAS PubMed.
- J. McNulty and C. Zepeda-Velázquez, Angew. Chem., 2014, 126, 8590–8594 CrossRef.
- S. Okumuş, C. Tanyeli and A. S. Demir, Tetrahedron Lett., 2014, 55, 4302–4305 CrossRef.
- Á. Martínez-Castañeda, K. Kędziora, I. Lavandera, H. Rodríguez-Solla, C. Concellón and V. del Amo, Chem. Commun., 2014, 50, 2598–2600 RSC.
- K. Higuchi, K. Masuda, T. Koseki, M. Hatori, M. Sakamoto and T. Kawasaki, Heterocycles, 2007, 73, 641–650 CrossRef CAS.
- Y.-Z. Liu, J. Zhang, P.-F. Xu and Y.-C. Luo, J. Inorg. Chem., 2011, 76, 7551–7555 CAS.
- S. Mahajan, P. Chauhan, C. C. Loh, S. Uzungelis, G. Raabe and D. Enders, Synthesis, 2015, 47, 1024 CrossRef CAS PubMed.
- T.-G. Chen, P. Fang, X.-L. Hou and L.-X. Dai, Synthesis, 2015, 47, 134–140 CrossRef CAS.
- W. Sun, L. Hong and R. Wang, Chem.–Eur. J., 2011, 17, 6030–6033 CrossRef CAS PubMed.
- M. Rueping, S. Raja and A. Núñez, Adv. Synth. Catal., 2011, 353, 563–568 CrossRef CAS.
- Y.-Z. Liu, R.-L. Cheng and P.-F. Xu, J. Org. Chem., 2011, 76, 2884–2887 CrossRef CAS PubMed.
- G. Talavera, E. Reyes, J. L. Vicario, L. Carrillo and U. Uria, Adv. Synth. Catal., 2013, 355, 653–658 CrossRef CAS.
- S. Belot, S. Sulzer-Mossé, S. Kehrli and A. Alexakis, Chem. Commun., 2008, 4694–4696 RSC.
- K. Huwig, K. Schultz and U. Kazmaier, Angew. Chem., Int. Ed., 2015, 54, 9120–9123 CrossRef CAS PubMed.
- R. Rios, I. Ibrahem, J. Vesely, H. Sundén and A. Córdova, Tetrahedron Lett., 2007, 48, 8695–8699 CrossRef CAS.
- P. Breistein, J. Johansson, I. Ibrahem, S. Lin, L. Deiana, J. Sun and A. Cordova, Adv. Synth. Catal., 2012, 354, 1156–1162 CrossRef CAS.
- F. Scorzelli, A. Di Mola, G. Croce, L. Palombi and A. Massa, Tetrahedron Lett., 2015, 56, 2787–2790 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedure and characterization data of the products. Copies of NMR spectra and HPLC analysis spectra of compounds 3, X-ray structural data (CIF) of compound 3aa and 3aa′. CCDC 1474611 and 1473998. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra10153d |
| This journal is © The Royal Society of Chemistry 2016 |