Mesoporous titanosilicate nanoparticles: facile preparation and application in heterogeneous epoxidation of cyclohexene†
Abstract
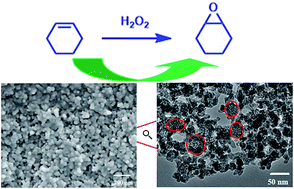
Mesoporous titanosilicate nanoparticles with a size of 40 to 75 nm (Nano-Ti-MCM-41) were hydrothermally synthesized from a titanosilicate (TiSil) solution with cetyltrimethylammonium bromide (CTAB) as the template and with a cationic polymer as the size-controlling agent. The particle size is proposed to be controlled by adjusting the size of TiSil-CTA+ micelles influenced by the charge interaction between negatively charged titanosilicate and polymer cationic ions during the formation of TiSil-CTA+ micelles. The presence of n-hexane and hydrogen peroxide in the preparation process proved to favor a well-ordered mesostructure in these nanoparticles: the mesostructure became disordered without using hexane and hydrogen peroxide although the size of mesoporous titanosilicate particles remained in the nanometer scale. The characterization results showed that Nano-Ti-MCM-41 possesses hierarchical porosity (bimodal mesopores) and an ordered mesoporous structure and that the titanium species are predominately located in tetrahedral framework positions. Nano-Ti-MCM-41 is a highly active catalyst for the epoxidation of cyclohexene with hydrogen peroxide, and displayed higher turnover numbers (TONs) based on the cyclohexene conversions and higher selectivity ratio between cyclohexene epoxide and 1,2-cyclohexanediol compared with traditional Ti-MCM-41 prepared without the cationic polymer. The improved catalytic performances are mainly ascribed to the decrease in the particle size of Ti-MCM-41, resulting in the enhanced accessibility of the reactants to the catalytic Ti species via the shorter channels of Nano-Ti-MCM-41 and in the shorter residence time of cyclohexene epoxide in the mesopores of the nanoparticles. Importantly, the mesoporous titanosilicate nanoparticles are stable catalysts immune to titanium leaching, suggesting their good recyclability potential in the epoxidation with aqueous H2O2.

 Please wait while we load your content...
Please wait while we load your content...