Sensor based on chemical vapour deposition-grown molybdenum disulphide for gas sensing application
Abstract
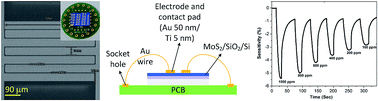
Over the past few decades, sensors based on field-effect transistors have drawn much attention. Initially three dimensional materials were used for sensing, which were later replaced by two dimensional materials because of their ease of manufacturing and large specific areas. Amongst the transition metal dichalcogenides, MoS2 has been widely used for the fabrication of sensors owing to its ability to differentiate between a charge donor and an acceptor analyte. In this work, we fabricated sensors using chemical vapour deposition grown-MoS2. MoS2 was grown on a p-Si/SiO2 substrate using Mo(CO)6 as a precursor, the growth was carried out by the sublimation of the precursor under a flow of high purity H2S at high temperature. The aim of this work is to achieve a level of sensitivity that would enable the detection of individual gas analytes upon adsorption to the MoS2 surface. To efficiently detect individual gas analytes upon adsorption to the surface, we used interdigitated electrodes in the device architecture to increase the area of the channels for analyte adsorption. We used CO2 and O2 gases, which acted as charge donors. A trilayer MoS2 film was examined, and the detection sensitivity for O2 was higher in comparison to CO2. The fabricated device showed significant sensitivity up to parts per million detection level.

 Please wait while we load your content...
Please wait while we load your content...