Layered double hydroxide/graphene oxide hybrid incorporated polysulfone substrate for thin-film nanocomposite forward osmosis membranes†
Peng Lua,
Shuai Lianga,
Tuantuan Zhoua,
Xueyi Meia,
Yu Zhanga,
Cheng Zhanga,
Ahmad Umarbc and
Qiang Wang*a
aCollege of Environmental Science and Engineering, Beijing Forestry University, 35 Qinghua East Road, Haidian District, Beijing 100083, P. R. China. E-mail: qiang.wang.ox@gmail.com; qiangwang@bjfu.edu.cn; Tel: +86-13699130626
bDepartment of Chemistry, College of Science and Arts, Najran University, Najran-11001, Kingdom of Saudi Arabia
cPromising Centre for Sensors and Electronic Devices (PCSED), Najran University, Najran-11001, Kingdom of Saudi Arabia
First published on 31st May 2016
Abstract
Herein, we report the use of a layered double hydroxide/graphene oxide (LDH/GO) hybrid as a nanofiller for a polysulfone (PSf) substrate in the fabrication of a thin film nanocomposite (TFN) forward osmosis (FO) membrane. The influence of the incorporation of the LDH/GO hybrid on the physicochemical properties of the PSf substrate was explored and a systematic investigation of the resultant TFN membrane performance was conducted. The results demonstrate that the addition of the LDH/GO hybrid enhanced the PSf substrate with increased porosity, hydrophilicity, surface pore diameter, and mechanical strength. Consequently, all the TFN membranes obtained increased water permeability and salt rejection, as compared to the thin film composite (TFC) membrane prepared on a conventional PSf substrate. Using 1 M NaCl as the draw solution and DI water as the feed solution, the water flux of the TFN membrane with a 2 wt% LDH/GO dosage as high as 23.6 L m−2 h−1 was obtained under the pressure retarded osmosis (PRO) mode. Compared to conventional TFC membranes, the TFN membrane with a 2 wt% LDH/GO showed a very low reverse salt flux (6.2 g m−2 h−1). The improvement in FO performance is attributed to the lower structural parameters of the modified PSf substrate, and the reduction of the internal concentration polarization. This study suggests the LDH/GO hybrid is an effective additive for modifying the PSf substrate for the development of FO membranes.
1. Introduction
Forward osmosis (FO) is a membrane-based separation process that has attracted considerable attention in recent years, and has often been regarded as one of the next generation technologies for water purification and seawater desalination.1–3 In an FO process, water molecules are spontaneously driven across a semipermeable membrane, due to the osmotic pressure gradient from the feed (low osmotic pressure) to the draw solution (high osmotic pressure).2 The driving force from the osmotic pressure in FO could be significantly higher than the hydraulic pressure in reverse osmosis (RO), suggesting a higher water flux in theory.4 However, internal concentration polarization (ICP), which is characterized by differing solute concentrations over the transverse boundaries of the support layer, occurs within the support layer of a membrane, leading to a reduction of the osmotic-pressure gradient across the active layer of the membrane, and thus a lower water flux.5,6 Therefore, the fabrication of high-performance FO membranes is an important goal in the FO research area.7–10A typical thin film composite (TFC) membrane used for FO applications consists of an ultra-thin polyamide (PA) active layer and a polysulfone (PSf) support layer.11 One recent important development in TFC membrane technology is the incorporation of nanomaterials into the PSf support layer to minimize the influence of ICP.12 The motivations for the choice of nanomaterials are manifold, and strongly depend on the size, type and amount of the nanomaterials used.13 To date, a range of inorganic nanoparticles, including metal oxide nanoparticles (e.g. TiO2 and SiO2),1,14–17 carbon-based nanomaterials (e.g. graphene oxide and CNTs)18–20 and other nanomaterials (e.g. zeolites and layered double hydroxides (LDHs))21–24 have been used in the fabrication of TFC FO membranes. For a FO membrane desalination process, these inorganic nanomaterials also help to enhance the permeability, mechanical and thermal stability of polymeric membranes. Emadzadeh et al.14 synthesized thin-film nanocomposite (TFN) membranes based on TiO2–PSf support layers for FO desalination. The results revealed that both the hydrophilicity and porosity of the substrate were increased by the addition of TiO2 nanoparticles. Similarly, SiO2 nanoparticles have also been demonstrated to be good modifying agents for enhancing water permeability, increasing the overall porosity, and reducing ICP of the PSf substrate.1 It is believed that the metal oxide nanoparticles could provide more water channels and/or high porosity.
Carbon-based nanomaterials are another type of promising material that can heighten the performance of polymeric membranes in desalination applications, and have experienced an upward trend in research in recent years.13 For instance, graphene oxide (GO), which contains abundant oxygen functional groups (carboxyl, epoxy and hydroxyl groups) and a one atomic thickness has been reported to offer high chemical stability, strong hydrophilicity, and excellent antifouling properties;20,25–27 a single-layer graphene membrane theoretically exhibits quick water transportation ability and a nearly 100% salt-rejection rate.28,29 Qin et al.30 incorporated GO into the support layer of a TFC membrane, which resulted in higher salt removals (>99.7% for multivalent ions). Park et al.20 fabricated a PSf/GO composite membrane support layer, which resulted in higher water flux and reverse-flux selectivity. In addition to the bare GO, functionalized GO nanoparticles as membrane fillers were also reported.31,32 For example, Chung's group utilized functionalized GO as a modifier to fabricate GO-based membranes. As a result, the newly developed membrane exhibited a high purity water permeability of 5.01 L m−2 h−1 bar−1, and comparably high rejections toward divalent metal ions. However, dried GO nanosheets may aggregate during preparation, which hinders the dispersion of GO nanosheets within the casting solution. In this work, we used LDHs as modifiers to prepare LDH/GO hybrid nanoparticles, in which the drying step was omitted and the dispersion of GO can be improved (Fig. S1†).
LDHs are a class of ionic lamellar compounds consisting of positively charged brucite-like layers, with interlayer spaces containing charged compensating anions and water molecules.33,34 Owing to their flexible, tunable chemical composition and high anion exchange capacity, LDHs have a huge advantage of being used as adsorbents, catalysts, and inorganic fillers.35–37 In our previous work,24 LDH nanoparticles were blended into the PSf substrate of a TFN membrane to increase porosity, hydrophilicity, mechanical strength, and thermal stability. The TFN membrane with the nanoparticle dosage of 2 wt% exhibited the highest water flux (∼42.5% higher than that of the pristine TFC membrane) with minor compromise of the reverse salt flux. In a recent review, Elimelech's group38 proposed that for next generation TFC membranes, it is also important to increase the selectivity, and not only the water permeability.
In this study, we prepared a LDH modified GO (LDH/GO) hybrid as a nanofiller for the PSf substrate of a TFN FO membrane. In principle, such an assembly is beneficial for maximizing the dispersions of both GO and LDH nanosheets. Moreover, GO nanosheets can be used to improve the membrane selectivity. A series of characterizations was conducted to understand the influence of LDH/GO on the structural properties and surface hydrophilicity of the PSf support layer. PSf substrates with varied LDH/GO loadings were prepared and tested to determine a more suitable support material composition for FO operation. To date, this work accounts for the first report on the TFN membrane using LDH/GO hybrid blended PSf substrates for FO applications.
2. Experimental
2.1. Synthesis of GO nanosheets
Graphite oxide was prepared by the modified Hummers' method, as described in our previous work.39,40 The exfoliated graphite oxide was dispersed in DI water, treated by ultra-sonication at 700 W for 1 h and then centrifuged at 8000 rpm for 8 min to remove any residual unexfoliated graphite oxide. The concentration of the delaminated graphite oxide dispersion, which was 1 g L−1, was determined by drying 100 mL of supernatant at 60 °C for two days, followed by measuring the mass using an electronic balance. The obtained sample was denoted as GO.2.2. In situ synthesis of LDH/GO nanomaterials
Briefly, 2.62 g of Mg(NO3)2·6H2O and 1.28 g of Al(NO3)3·9H2O were dissolved in 100 mL of deionized water (solution A). Another 100 mL solution containing 0.18 g of Na2CO3 and 70 mL of GO suspension (solution B) was prepared. Solution A was added to solution B under vigorous stirring at pH 10 (strictly controlled using a 4 M NaOH solution). The resulting mixture was aged at room temperature for 12 h under continuous stirring. Afterwards, the obtained LDH/GO hybrid was washed with H2O until pH 7 was reached, and it was further intensively washed with acetone to prevent LDH/GO hybrid agglomeration.34 The obtained LDH/GO slurry was directly used for the preparation of PSf substrates.2.3. Preparation of LDH/GO, LDH, and GO incorporated PSf substrates
LDH/GO, LDH, and GO incorporated PSf (Mn: 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, Sigma-Aldrich) substrates were fabricated by a phase inversion technique. The chemical compositions of casting solutions are shown in Table 1. A certain amount of inorganic nanofillers was first added to the NMP/DMF mixture, followed by a 1 h ultra-sonication to minimize the agglomeration of inorganic nanofillers. The specific preparation process for the PSf substrates was reported in our previous work.24 The LDH/GO hybrid incorporated PSf substrates were designated as PSf, PSf-1, PSf-2, PSf-3, PSf-4, which correspond to the LDH/GO hybrid loading of 0, 1, 2, 3, and 4 wt%, respectively. The LDH and GO incorporated PSf substrates were designated as PSf–LDH and PSf–GO, respectively.
000 Da, Sigma-Aldrich) substrates were fabricated by a phase inversion technique. The chemical compositions of casting solutions are shown in Table 1. A certain amount of inorganic nanofillers was first added to the NMP/DMF mixture, followed by a 1 h ultra-sonication to minimize the agglomeration of inorganic nanofillers. The specific preparation process for the PSf substrates was reported in our previous work.24 The LDH/GO hybrid incorporated PSf substrates were designated as PSf, PSf-1, PSf-2, PSf-3, PSf-4, which correspond to the LDH/GO hybrid loading of 0, 1, 2, 3, and 4 wt%, respectively. The LDH and GO incorporated PSf substrates were designated as PSf–LDH and PSf–GO, respectively.
| FO substrates | Compositions of casting solutions | |||
|---|---|---|---|---|
| PSf (wt%) | NMP (wt%) | DMF (wt%) | Nanofillersa (wt%) | |
| a The mass of added LDH, GO, and LDH/GO hybrid was based on the total mass of polymers. | ||||
| PSf | 12 | 66 | 22 | 0 |
| PSf-1 | 12 | 66 | 22 | 1 |
| PSf-2 | 12 | 66 | 22 | 2 |
| PSf-3 | 12 | 66 | 22 | 3 |
| PSf-4 | 12 | 66 | 22 | 4 |
| PSf–GO | 12 | 66 | 22 | 2 |
| PSf–LDH | 12 | 66 | 22 | 2 |
2.4. Preparation of the PA active layer
The PA active layer was prepared via interfacial polymerization between 1,3-phenylenediamine (MPD, >99%) and 1,3,5-benzenetricarbonyl trichloride (TMC, ∼98%) on the surface of the PSf substrates as described in our previous work.24 The fabricated TFC membranes were thoroughly rinsed with DI water and stored in a deionized water bath at 4 °C. These obtained membranes, based on the LDH/GO hybrid incorporated PSf substrates, are denoted as TFC (PA@PSf), TFN-1 (PA@PSf-1), TFN-2 (PA@PSf-2), TFN-3 (PA@PSf-3), and TFN-4 (PA@PSf-4). The number in the designation represents the dosage of the LDH/GO hybrid in the substrates. The TFN membranes based on the GO or LDH nanosheets incorporated PSf substrates were denoted as TFN–GO and TFN–LDH, respectively.2.5. LDH/GO hybrid and membrane characterizations
X-ray diffraction (XRD) was conducted with a Shimadzu XRD-7000 instrument, with Cu Kα radiation at a power of 40 kV × 40 mA, in the scanning angle (2θ) range of 5–80°, with a step size of 0.02°. TEM analysis was carried out using a JEOL JEM-1010 microscope with an accelerating voltage of 80 kV. Samples were dispersed in ethanol and deposited onto a perforated carbon foil supported on a copper grid. Membrane morphologies were observed with a field emission scanning electron microscope (FE-SEM, SU8010 Hitachi). Before observation, membranes were freeze-dried and fractured in liquid nitrogen, and then sputter-coated with gold. Elemental compositions of the surfaces of the substrates were determined by energy dispersive spectrometry (EDS) with a 10 keV energy beam. Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR, Bruker VERTEX 70) was used to identify the functional groups of the polyamide active layer and analyze the chemical changes of the PSf substrates. Surface roughness was measured by atomic force microscopy (AFM, NanoScope V MultiMode8, Bruker). A tapping mode was operated at room temperature, and imaged in the range of 5 μm × 5 μm. Surface hydrophilicity of the PSf substrates was evaluated by water contact angle, using the sessile drop method (Dataphysics OCA20). For each membrane type, three different samples were tested in twelve random locations. Membrane wettability was evaluated by calculating the solid–liquid interfacial free energy with the following eqn (1):41,42
 | (1) |
 | (2) |
2.6. Evaluation of membrane performance
The pure water flux of PSf–LDH/GO substrates was measured using a dead-end filtration device with an effective membrane area of 38.47 cm2 under a pressure of 1 bar. The schematic diagram of the lab-scale FO system and experimental setup are described in our previous work.24 Water flux (Jw) and reverse salt flux (Js) are parameters for evaluating membrane performance. The obtained membranes were tested at two different modes: (1) draw solution facing the PA active layer (PRO mode), and (2) feed water facing the PA active layer (FO mode). Each test was conducted for 1 h in triplicate.2.7. Determination of the transport and structural parameters
The water permeability coefficient (A), salt permeability coefficient (B), and structural parameter (S) of the FO membranes were determined with the excel-based algorithm developed by Tiraferri et al.43 A/B was termed the reverse flux selectivity, and regarded as a quality control parameter.44 Non-linear regression was performed on the bases of eqn (3) and (4), with each stage corresponding to a special draw solution concentration:
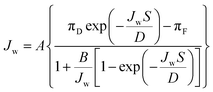 | (3) |
 | (4) |
3. Results and discussion
3.1. Characterizations of the LDH/GO nanoparticles
Fig. 1(a) depicts the XRD pattern of the dried GO, LDH, and LDH/GO hybrid. The characteristic diffraction peak of the exfoliated GO observed at 11.8° can be associated with the reflection of the (002) plane, which has the interlayer space of 0.75 nm, owing to the introduction of the oxygen-containing functional groups, water molecules, and other molecules after oxidation.37,39,45 For the LDH, the observed six characteristic peaks could be indexed to the brag reflections of the (003), (006), (009), (015), (018), and (110/113) planes, respectively, which suggest a well-developed layered structure.46 For the LDH/GO hybrid, no obvious differences can be found in the XRD pattern after the addition of GO, suggesting that the GO addition did not affect the layered structure of LDH. The ATR-FTIR profiles of GO, LDH and the LDH/GO hybrid are displayed in Fig. 1(b). The sharp peak at 1370 cm−1 is ascribed to a strong interaction of the carbonate anions within the layer. The bands appearing at 655–584 cm−1 are attributed to the Al–O and Mg–O vibrations.37,39 For the GO profile, a band at 1717 cm−1 due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, a band at 1224 cm−1 corresponding to –COOH and C–O–C stretching, and a band at 1410 cm−1 attributed to the stretching of O–H groups were detected, which correspond well to its structure.32,45 In the LDH/GO profile, the peaks at 1085 and 1049 cm−1 could be attributed to the vibrations of oxygen-containing functional groups, which suggest the presence of GO.45,47
O stretching vibration, a band at 1224 cm−1 corresponding to –COOH and C–O–C stretching, and a band at 1410 cm−1 attributed to the stretching of O–H groups were detected, which correspond well to its structure.32,45 In the LDH/GO profile, the peaks at 1085 and 1049 cm−1 could be attributed to the vibrations of oxygen-containing functional groups, which suggest the presence of GO.45,47
The morphologies of the LDH/GO hybrids were examined via FE-SEM and HR-TEM. The SEM image (Fig. 1(c)) of the dried LDH/GO powder clearly indicates a typical flower-like morphology; however, severe aggregation was observed because of the high surface charge and surface tension of the nanoparticles in the dry conditions.34,35 Fig. 1(d) exhibits the HR-TEM images of the obtained LDH/GO hybrid; both LDH and GO nanosheets are clearly observed (indicated by arrows). The nano-sized LDH platelets randomly grow on the GO nanosheets. Some LDHs grow with ab-planes of the crystallites parallel to the graphene surface, whereas some LDHs grow with ab-planes perpendicular to the graphene surface.48 The average particle size of the LDH/GO nanosheets ranges from 100 to 150 nm. Fig. S2† shows the plane view of the HR-TEM image with ×50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 amplification of LDH/GO hybrid nanosheets, from which the LDH crystal lattice fringe could be plainly observed.
000 amplification of LDH/GO hybrid nanosheets, from which the LDH crystal lattice fringe could be plainly observed.
3.2. Properties of the LDH/GO hybrid incorporated PSf substrates
The LDH/GO incorporated PSf substrates were first characterized by XRD analysis, as shown in Fig. 2. A weak peak can be clearly observed at ∼11.3°, verifying the existence of LDH/GO in the PSf substrates. This result is consistent with the reported literature.24 The morphology of the synthesized nanocomposite substrates was further examined using SEM analysis. Fig. 3 presents the top surfaces of the LDH/GO-incorporated PSf substrates. The incorporation of the LDH/GO hybrid induced a slight difference in the surface morphology of the PSf substrates. The average surface pore diameter was significantly increased with the incorporation of the LDH/GO hybrid. Consequently, the PSf surface became more open, thus providing minor resistance for mass transport.49 The existence of the LDH/GO hybrid in the substrate membranes was further confirmed by the EDX results (Fig. 3(f)). With the increase of the nanofiller loading, the aggregation of the LDH/GO hybrid was aggravated, as shown in Fig. 3(d) and (e); the EDX mapping (Fig. S3†) confirmed this phenomenon.To further study the structures of the five different substrates with incorporation of the LDH/GO hybrid, the cross-sections of the substrates were also explored. Fig. 4(a) shows a typical structure with finger-like pores near the membrane surface, which opens up to larger macrovoids towards the bottom.23 By increasing the loading of the LDH/GO (Fig. 4(b)–(e)), the two-layer structure can be observed more and more apparently, with one finger-like layer near the surface and a second layer near the bottom, which consists of both ellipsoidal macrovoids and a sponge-like structure. This morphology change could be ascribed to the addition of the hydrophilic nanoparticles.24 The presence of the hydrophilic LDH/GO hybrid in the casting solution strongly promotes the diffusion of water from the water coagulation bath to the cast polymer film, leading to the development of larger macrovoids and the enhancement in the overall porosity.17,24 In general, the LDH/GO hybrid incorporated PSf substrates possessed larger overall porosity than the pristine PSf substrate (Table 3). This type of open surface morphology and cross-section structure might be beneficial in reducing ICP and thus enhancing water permeability for the resultant membrane.21,24
 | ||
| Fig. 4 SEM images of the cross section of the PSf substrates with different LDH/GO hybrid loadings: (a) PSf, (b) PSf-1, (c) PSf-2, (d) PSf-3, (e) PSf-4, and (f) local view in PSf-4. | ||
Table 2 summarizes the effects of the LDH/GO hybrid, incorporating the properties of PSf substrates, regarding thickness, pure water permeability, overall porosity, contact angle, and interfacial free energy. Compared to the pristine PSf substrate, the pure water permeability of the PSf-2 substrate was increased from 625 to 783 L m−2 h−1 bar−1. The LDH/GO hybrid incorporation had a positive effect on the increase of the substrate permeability.19 In addition, the overall porosity was significantly increased (see Table 2), due to the incorporation of the LDH/GO hybrid, which might be a key factor resulting in the smaller structure (S value), considering the S value is inversely proportional to membrane porosity.17 However, more LDH/GO hybrid did not result in a further increase in the permeability and overall porosity, due to the difficulty in achieving a uniform LDH/GO dispersion. Furthermore, the thickness of the PSf substrates decreased with the increase of the LDH/GO loading. This is because the addition of the LDH/GO hybrid made the substrates denser; a similar phenomenon was observed in the previously reported studies as well.1,20,21 In general, a smaller S value suggests a better performance of the support layer in minimizing ICP during the FO process, consequently leading to higher water permeability.16
| PSf substrates | Thickness (μm) | Pure water flux (L m−2 h−1 bar−1) | Overall porosity (%) | Contact angle (deg) | −ΔGML (mJ m−2) |
|---|---|---|---|---|---|
| PSf | 66.8 ± 1.8 | 625 | 75.6 ± 1.3 | 84.7 ± 1.9 | 79.5 |
| PSf-1 | 63.7 ± 1.3 | 678 | 81.8 ± 1.6 | 82.6 ± 1.4 | 80.7 |
| PSf-2 | 58.6 ± 2.1 | 783 | 82.2 ± 0.2 | 81.9 ± 2.1 | 82.4 |
| PSf-3 | 53.5 ± 1.7 | 717 | 82.1 ± 1.8 | 77.4 ± 2.6 | 85.7 |
| PSf-4 | 50.6 ± 0.5 | 660 | 81.3 ± 0.1 | 74.7 ± 2.9 | 86.3 |
With the addition of the hydrophilic LDH/GO hybrid, the hydrophilicity of PSf substrates was partly improved. The contact angle decreased from 84.7° to 74.7° for the pristine PSf and PSf-4 membranes, respectively. Next, the interfacial free energy of the membranes was analyzed using eqn (1).41 The pristine PSf membrane was found to be relatively wetted (−ΔGML = 79.5 mJ m−2) when immersed in deionized water. Moreover, the interfacial free energy increased from 79.5 to 86.3 mJ m−2 along with the increase of LDH/GO loading. This increase is due to the increase of both the Lifshitz–van der Waals and Lewis acid–base components.41 Based on the acquired data, the LDH/GO loading could be a significant factor that improved the properties, such as the hydrophilicity, the overall porosity, and the water permeability of the support layer.
Fig. 5 shows the mechanical properties of the membrane substrates with varying amounts of LDH/GO. Both the tensile strength and elongation-at-break increased as the LDH/GO loading increased, suggesting the enhancement effect of the LDH/GO hybrid on membrane mechanical strength. The elongation-at-break of the substrate membrane was slightly increased with low dosages of the LDH/GO hybrid. However, when the LDH/GO loading was increased over 2 wt%, a dramatic elongation-at-break increase was observed. This improvement may be ascribed to a possible rearrangement of the GO and/or LDH platelets, which resulted in a greater deformation,50 thereby leading to an increase in membrane strength. In addition, the tensile strength was slightly increased owing to the addition of the LDH/GO hybrid.
3.3. Effect of the LDH/GO hybrid on the properties of TFC FO membranes
The ATR-FTIR spectra of the PSf substrate, PSf–LDH/GO nanocomposite substrates, and TFC membranes are presented in Fig. 6. The significant peaks at 1150 (symmetric O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1293 (asymmetric O
O stretching), 1293 (asymmetric O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1240 (asymmetric C–O–C stretching), 1490 (CH3–C–CH3 stretching), and 1408 cm−1 (C
O stretching), 1240 (asymmetric C–O–C stretching), 1490 (CH3–C–CH3 stretching), and 1408 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic ring stretching) correspond to the specific groups of PSf.14,51,52 The detailed spectra in the range from 2000 to 400 cm−1 (Fig. 6(b)) reveal a significant change in the weak peak at ∼620 cm−1, which gradually became stronger with the increase in LDH/GO loading. This peak is associated with the Al–O and Mg–O vibrations of the LDH/GO hybrid. These results verified the presence of the LDH/GO hybrid in the substrates. A comparison of the PSf substrate and the TFC membrane spectra disclosed the characteristic peaks of the PA layer formed by the interfacial polymerization of MPD and TMC monomers at 1662, 1610, and 1541 cm−1, indicating the successful formation of the PA layer on top of the PSf substrate. The peaks were attributed to the aromatic ring breathing, amide I band (C
C aromatic ring stretching) correspond to the specific groups of PSf.14,51,52 The detailed spectra in the range from 2000 to 400 cm−1 (Fig. 6(b)) reveal a significant change in the weak peak at ∼620 cm−1, which gradually became stronger with the increase in LDH/GO loading. This peak is associated with the Al–O and Mg–O vibrations of the LDH/GO hybrid. These results verified the presence of the LDH/GO hybrid in the substrates. A comparison of the PSf substrate and the TFC membrane spectra disclosed the characteristic peaks of the PA layer formed by the interfacial polymerization of MPD and TMC monomers at 1662, 1610, and 1541 cm−1, indicating the successful formation of the PA layer on top of the PSf substrate. The peaks were attributed to the aromatic ring breathing, amide I band (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and amide II band (C–H stretching), respectively.14,22,51,53
O stretching) and amide II band (C–H stretching), respectively.14,22,51,53
The surface morphology and surface roughness of the TFC membrane were analyzed using FE-SEM and AFM (Fig. 7). The SEM images of both TFC and TFN membranes showed rough surfaces with a nodular structure. Meanwhile, the corresponding AFM images exhibited a “ridge-valley” structure throughout the plane.24,54 Along with the increase of the LDH/GO loading, the average surface roughness (Ra) showed a consecutive increase from 79.1 to 101 nm. The Ra increase might be attributed to the water flux enhancement, due to the increase of the filtration area for water transport.17
 | ||
| Fig. 7 (a)–(e) SEM and (f)–(j) 3D-AFM images of the top surfaces of the TFN prepared with different LDH/GO dosages. | ||
3.4. Effect of the LDH/GO hybrid on the FO performance
Fig. 8 compares the FO and PRO performance of the TFN membranes using 1 M NaCl as the draw solution and deionized water as the feed solution. Generally, all the membranes possessed a much higher water flux in the PRO configuration than that in the FO configuration. This result indicates that the degree of the concentrative/dilutive ICPs in the two membrane orientations is different, which was well elucidated in previous studies.5,8 As can be observed in Fig. 8(a), with an approximate 2 wt% LDH/GO loading, the average water flux of the TFN-2 membrane was significantly improved from 8.6 to 13.4 L m−2 h−1 in the FO orientation, and from 15.3 to 23.6 L m−2 h−1 in the PRO orientation. These results are in good agreement with the above SEM and AFM analyses (see Fig. 3, 4 and 7). The LDH/GO loading was increased and the water flux of TFN-3 and TFN-4 were not significantly changed in the FO orientation. However, the water flux of TFN-3 and TFN-4 were decreased slightly in the PRO orientation, which might be due to the aggregation of the LDH/GO hybrid when its loading was high.Fig. 8(b) presents the reverse salt flux of membranes tested at FO and PRO orientations, which slowly increased following the water flux improvement from the TFN membranes. Surprisingly, the best performing TFN-2 membrane was able to provide an average Js of 6.2 g m−2 h−1 in the FO orientation and Js of 6.9 g m−2 h−1 in the PRO orientation. Compared to the TFC membrane, the TFN-2 membrane possessed an 8% lower reverse salt flux in the FO orientation and 3% lower in the PRO orientation. This phenomenon might be due to the addition of GO, which exhibited a higher salt rejection rate.29,45 Furthermore, Jw/Js is known as the reverse flux selectivity, and serves as a quality control parameter (Fig. 8(c)).44 A higher Jw/Js value for TFN-2 indicates that the LDH/GO hybrid would be conducive to the reverse solute selectivity, which is one of the most important factors for membrane design.54 Based on the results obtained from the osmotic test, it can be concluded that 2 wt% is the optimal LDH/GO loading for the PSf–LDH/GO substrates, which results in higher water flux and salt rejection. In addition, in order to prove the superiority of the LDH/GO hybrid, a 2 wt% loading of GO or LDH nanosheets was blended into the PSf substrates for the preparation of TFN membranes. The TFN–LDH membrane showed the same phenomenon in our previous work, where both water flux and reverse salt flux were increased.24 The TFN–GO membrane exhibited better rejection of salt. However, the water flux of TFN–GO membrane was much lower, which maybe because the dispersion of GO nanosheet was poor in the casting solution (see Fig. S1†). Among these three types of TFN membranes, TFN-2 exhibited higher water flux and better selectivity. Table 3 presents a summary of the performance of TFN membranes in this work, and commercial CTA membranes from Hydration Technology Innovations (HTI).55
| Membranes | Water flux (L m−2 h−1) FO/PRO | Reverse salt flux (g m−2 h−1) FO/PRO | Feed solution | Draw solution | References |
|---|---|---|---|---|---|
| a One of the FO membranes was cut from a Hydrowell® module (denoted as CTA-HW), the other two membranes were received as flat coupons from Hydration Technology Innovations (HTI), either supported by a polyester woven fabric (denoted as CTA-W) or a non-woven fabric (denoted as CTA-NW). | |||||
| TFC | 8.6/15.3 | 6.8/7.2 | DI water | 1 M NaCl | In this work |
| TFN-2 | 13.4/23.6 | 6.2/6.9 | DI water | 1 M NaCl | In this work |
| TFN–GO | 9.2/18.0 | 3.8/6.4 | DI water | 1 M NaCl | In this work |
| TFN–LDH | 9.3/22.3 | 6.5/11.4 | DI water | 1 M NaCl | In this work |
| CTA-HWa | 9.03/15.4 | 5.3/9.4 | 10 mM NaCl | 0.5 M NaCl | 55 |
| CTA-Wa | 5.0/6.55 | 2.9/4.8 | 10 mM NaCl | 0.5 M NaCl | 55 |
| CTA-NWa | 4.4/8.19 | 0.6/2.8 | 10 mM NaCl | 0.5 M NaCl | 55 |
To further clarify the effect of the LDH/GO hybrid on the permeability of the TFN membranes, water permeability (A), salt permeability (B), and structural parameters (S) were calculated on the basis of the results of water flux and reverse salt flux in the FO tests.43,44 The predicted transport and structural parameters of the TFC and TFN membranes are shown in Table 4. The TFN-2 membrane with 2 wt% LDH/GO substrate had a higher water permeability than the TFC membrane. Simultaneously, the salt permeability of TFN-2 was significantly reduced. This phenomenon shows that LDH/GO increased the selectivity of the TFC membranes. The low S value of a membrane means minor ICP in the FO process. The TFN-2 membrane has a low S value of 138 μm, which is desirable for FO membranes. A higher A/B value for TFN-2 indicates that the LDH/GO would contribute to the reverse solute selectivity. All the observations confirm that the LDH/GO hybrid blending of the PSf substrate matrix is an effective method to reduce the S value, and the TFN membrane based on the PSf–LDH/GO has great potential for FO desalination.
| Membranes | A (L m−2 h−1 bar−1) | B (L m−2 h−1) | S (μm) | A/B (1/bar) |
|---|---|---|---|---|
| TFC | 0.48 | 0.22 | 287 | 2.16 |
| TFN-1 | 0.51 | 0.16 | 169 | 3.19 |
| TFN-2 | 0.53 | 0.15 | 138 | 3.53 |
| TFN-3 | 0.56 | 0.19 | 167 | 2.95 |
| TFN-4 | 0.54 | 0.32 | 150 | 1.69 |
4. Conclusions
A LDH/GO hybrid was synthesized and incorporated into a PSf substrate for the preparation of the TFN FO membrane. Within the LDH/GO dosage range (0–4 wt%), the hydrophilicity, overall porosity, surface pore diameter, and mechanical strength of the nanocomposite support layer were dramatically improved. The incorporation of the LDH/GO hybrid into the PSf layer, which led to a significant change of the cross-sectional structure, was examined using XRD, ATR-FTIR, EDS, and SEM analyses. Furthermore, the water flux of the TFN membrane was also increased by using the PSf–LDH/GO nanocomposite substrates, without compromising the reverse salt flux. These improvements could be attributed to the changes in membrane structure that are favorable for ICP alleviation, thus enhancing water permeability. Our results suggest that the modification strategy with the LDH/GO hybrid is a promising approach for the fabrication of a high performance FO membrane.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (2016ZCQ03), the National Natural Science Foundation of China (51572029, 51308045), and the Beijing Nova Programme (Z131109000413013).References
- X. Liu and H. Y. Ng, J. Membr. Sci., 2015, 481, 148–163 CrossRef CAS.
- P. Lu, Y. Gao, A. Umar, T. Zhou, J. Wang, Z. Zhang, L. Huang and Q. Wang, Sci. Adv. Mater., 2015, 7, 2182–2192 CrossRef CAS.
- N. Widjojo, T. S. Chung, M. Weber, C. Maletzko and V. Warzelhan, Chem. Eng. J., 2013, 220, 15–23 CrossRef CAS.
- N. Y. Yip, A. Tiraferri, W. A. Phillip, J. D. Schiffman and M. Elimelech, Environ. Sci. Technol., 2010, 44, 3812–3818 CrossRef CAS PubMed.
- G. T. Gray, J. R. McCutcheon and M. Elimelech, Desalination, 2006, 197, 1–8 CrossRef CAS.
- Z. Z. Zhou, J. Y. Lee and T. S. Chung, Chem. Eng. J., 2014, 249, 236–245 CrossRef CAS.
- S. F. Zhao, L. Zou, C. Y. Y. Tang and D. Mulcahy, J. Membr. Sci., 2012, 396, 1–21 CrossRef CAS.
- T. Y. Cath, A. E. Childress and M. Elimelech, J. Membr. Sci., 2006, 281, 70–87 CrossRef CAS.
- T. S. Chung, S. Zhang, K. Y. Wang, J. C. Su and M. M. Ling, Desalination, 2012, 287, 78–81 CrossRef CAS.
- N. Niksefat, M. Jahanshahi and A. Rahimpour, Desalination, 2014, 343, 140–146 CrossRef CAS.
- D. Stillman, L. Krupp and Y.-H. La, J. Membr. Sci., 2014, 468, 308–316 CrossRef CAS.
- W. J. Lau, A. F. Ismail, N. Misdan and M. A. Kassim, Desalination, 2012, 287, 190–199 CrossRef CAS.
- P. S. Goh and A. F. Ismail, J. Chem. Technol. Biotechnol., 2015, 90, 971–980 CrossRef CAS.
- D. Emadzadeh, W. J. Lau and A. F. Ismail, Desalination, 2013, 330, 90–99 CrossRef CAS.
- D. Emadzadeh, W. J. Lau, T. Matsuura, N. Hilal and A. F. Ismail, Desalination, 2014, 348, 82–88 CrossRef CAS.
- D. Emadzadeh, W. J. Lau, T. Matsuura, A. F. Ismail and M. Rahbari-Sisakht, J. Membr. Sci., 2014, 449, 74–85 CrossRef CAS.
- D. Emadzadeh, W. J. Lau, T. Matsuura, M. Rahbari-Sisakht and A. F. Ismail, Chem. Eng. J., 2014, 237, 70–80 CrossRef CAS.
- X. J. Song, L. Wang, C. Y. Tang, Z. N. Wang and C. J. Gao, Desalination, 2015, 369, 1–9 CrossRef CAS.
- Y. Q. Wang, R. W. Ou, H. T. Wang and T. W. Xu, J. Membr. Sci., 2015, 475, 281–289 CrossRef CAS.
- M. J. Park, S. Phuntsho, T. He, G. M. Nisola, L. D. Tijing, X.-M. Li, G. Chen, W.-J. Chung and H. K. Shon, J. Membr. Sci., 2015, 493, 496–507 CrossRef CAS.
- N. Ma, J. Wei, S. R. Qi, Y. Zhao, Y. B. Gao and C. Y. Y. Tang, J. Membr. Sci., 2013, 441, 54–62 CrossRef CAS.
- W. kuang, Z. Liu, H. Yu, G. Kang, X. Jie, Y. Jin and Y. Cao, J. Membr. Sci., 2016, 497, 485–493 CrossRef CAS.
- G. D. Vilakati, M. C. Y. Wong, E. M. V. Hoek and B. B. Mamba, J. Membr. Sci., 2014, 469, 216–224 CrossRef CAS.
- P. Lu, S. Liang, L. Qiu, Y. Gao and Q. Wang, J. Membr. Sci., 2016, 504, 196–205 CrossRef CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- P. S. Goh and A. F. Ismail, Desalination, 2015, 356, 115–128 CrossRef CAS.
- K. Goh, L. Setiawan, L. Wei, R. Si, A. G. Fane, R. Wang and Y. Chen, J. Membr. Sci., 2015, 474, 244–253 CrossRef CAS.
- S. P. Surwade, S. N. Smirnov, I. V. Vlassiouk, R. R. Unocic, G. M. Veith, S. Dai and S. M. Mahurin, Nat. Nanotechnol., 2015, 10, 459–464 CrossRef CAS PubMed.
- X. Zhang and J.-G. Gai, RSC Adv., 2015, 5, 68109–68116 RSC.
- D. Qin, Z. Liu, D. Delai Sun, X. Song and H. Bai, Sci. Rep., 2015, 5, 14530 CrossRef CAS PubMed.
- Y. P. Tang, J. X. Chan, T. S. Chung, M. Weber, C. Staudt and C. Maletzko, J. Mater. Chem. A, 2015, 3, 10573–10584 CAS.
- Y. Zhang, S. Zhang and T. S. Chung, Environ. Sci. Technol., 2015, 49, 10235–10242 CrossRef CAS PubMed.
- Q. Wang, X. Zhang, J. Zhu, Z. Guo and D. O'Hare, Chem. Commun., 2012, 48, 7450–7452 RSC.
- Q. Wang and D. O'Hare, Chem. Commun., 2013, 49, 6301–6303 RSC.
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS PubMed.
- Y. F. Zhao, B. Li, Q. Wang, W. Gao, C. L. J. Wang, M. Wei, D. G. Evans, X. Duan and D. O'Hare, Chem. Sci., 2014, 5, 951–958 RSC.
- J. Y. Wang, X. Y. Mei, L. Huang, Q. W. Zheng, Y. Q. Qiao, K. Zang, S. Mao, R. Y. Yang, Z. Zhang, Y. S. Gao, Z. H. Guo, Z. Huang and Q. Wang, J. Energy Chem., 2015, 24, 127–137 CrossRef.
- J. R. Werber, A. Deshmukh and M. Elimelech, Environ. Sci. Technol. Lett., 2016, 3, 112–120 CrossRef CAS.
- X. Mei, J. Wang, R. Yang, Q. Yan and Q. Wang, RSC Adv., 2015, 5, 78061–78070 RSC.
- S. Sahebi, S. Phuntsho, Y. C. Woo, M. J. Park, L. D. Tijing, S. Hong and H. K. Shon, Desalination, 2015, 389, 129–136 CrossRef.
- S. Liang, Y. Kang, A. Tiraferri, E. P. Giannelis, X. Huang and M. Elimelech, ACS Appl. Mater. Interfaces, 2013, 5, 6694–6703 CAS.
- A. Tiraferri, C. D. Vecitis and M. Elimelech, ACS Appl. Mater. Interfaces, 2011, 3, 2869–2877 CAS.
- A. Tiraferri, N. Y. Yip, A. P. Straub, S. R. V. Castrillon and M. Elimelech, J. Membr. Sci., 2013, 444, 523–538 CrossRef CAS.
- Y. Wang, X. Li, C. Cheng, Y. He, J. Pan and T. Xu, J. Membr. Sci., 2016, 498, 30–38 CrossRef CAS.
- Z. Lv, X. Yang and E. Wang, Nanoscale, 2013, 5, 663–670 RSC.
- Y. Gao, J. Wu, Z. Zhang, R. Jin, X. Zhang, X. Yan, A. Umar, Z. Guo and Q. Wang, J. Mater. Chem. A, 2013, 1, 9928 CAS.
- M. Hu and B. Mi, Environ. Sci. Technol., 2013, 47, 3715–3723 CrossRef CAS PubMed.
- S. Huang, G. N. Zhu, C. Zhang, W. W. Tjiu, Y. Y. Xia and T. Liu, ACS Appl. Mater. Interfaces, 2012, 4, 2242–2249 CAS.
- X. Lu, L. H. Arias Chavez, S. Romero-Vargas Castrillon, J. Ma and M. Elimelech, Environ. Sci. Technol., 2015, 49, 1436–1444 CrossRef CAS PubMed.
- P. Anadão, L. F. Sato, H. Wiebeck and F. R. Valenzuela-Díaz, Appl. Clay Sci., 2010, 48, 127–132 CrossRef.
- Y. Huang, H. Jin, P. Yu and Y. Luo, Desalin. Water Treat., 2015, 56, 1–11 CrossRef.
- D. L. Shaffer, H. Jaramillo, S. R. V. Castrillon, X. L. Lu and M. Elimelech, J. Membr. Sci., 2015, 490, 209–219 CrossRef CAS.
- N. Ma, J. Wei, R. H. Liao and C. Y. Y. Tang, J. Membr. Sci., 2012, 405, 149–157 CrossRef.
- D. Emadzadeh, W. J. Lau, M. Rahbari-Sisakht, H. Ilbeygi, D. Rana, T. Matsuura and A. F. Ismail, Chem. Eng. J., 2015, 281, 243–251 CrossRef CAS.
- J. Wei, C. Q. Qiu, C. Y. Y. Tang, R. Wang and A. G. Fane, J. Membr. Sci., 2011, 372, 292–302 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10080e |
| This journal is © The Royal Society of Chemistry 2016 |






