Encapsulation of honokiol-loaded nanoparticles in lecithin microbubbles for targeted tumor therapy
Zhen Lia,
Lan Haob,
Pei Yuana,
Wenjing Huc and
Liangke Zhang*a
aChongqing Key Laboratory of Biochemistry and Molecular Pharmacology, Chongqing Research Center for Pharmaceutical Engineering, School of Pharmacy, Chongqing Medical University, District of Yuzhong, Chongqing 400016, P. R. China. E-mail: zlkdyx@126.com; Fax: +86-23-6848-5161; Tel: +86-23-6848-5161
bChongqing Key Laboratory of Ultrasound Molecular Imaging, Institute of Ultrasound Imaging, Chongqing Medical University, Chongqing 400016, P. R. China
cChongqingshi Shapingba District People's Hospital, Chongqing 400030, P. R. China
First published on 1st June 2016
Abstract
This study aimed to develop a new drug delivery system that combines honokiol-loaded albumin nanoparticles (HKNs) with perfluorocarbon-filled microbubbles (MBs) to improve the target delivery of honokiol (HK). The HKNs were prepared through a desolvation method. The MBs were prepared by mechanical shaking and used to encapsulate the HKNs and perfluorocarbon gas (HKN-MBs). The prepared HKN-MBs displayed a mean diameter of 1447.0 ± 182.4 nm and a drug-loading efficiency of 3.48% ± 0.20%. The HKN-MBs demonstrated good acoustic properties in in vitro and in vivo ultrasound (US) imaging experiments, in which no significant difference to traditional MBs was found. The HKN-MBs had high drug encapsulation and loading efficiency while maintaining the acoustic properties as an US contrast agent. C57BL/6 mice with Lewis lung carcinoma tumor were treated with HKN-MBs through the tail vein and then exposed to an US destruction pulse, resulting in an improved curative effect on the tumor tissues. The experimental group administered with HKN-MBs and exposed to US showed an obvious increase in the rate of tumor growth inhibition (48.78%) compared with those of HKN-MBs (32.85%) and HKNs (29.94%). Encapsulation of HKNs in MBs is expected to improve the therapeutic efficacy of HK and provide a feasible technique for targeted tumor therapy.
Introduction
Honokiol (HK) is a bioactive lignanoid extracted from the reed of Magnolia officinalis that has been utilized in the treatment of thrombotic stroke, gastrointestinal complaints, anxiety, nervous disturbance, and cancer.1–3 Previous contributions have reported that HK exhibits anticancer properties, but its poor aqueous solubility limits its clinical applications.Gas-filled microbubbles (MBs) have been used as ultrasound (US) contrast agents for many years.4–6 MBs are micrometer-sized gaseous particles stabilized by a lipid monolayer shell.7 In recent years, US-induced MB destruction has evolved into a new tool for drug delivery.8,9 Acoustic imaging allows real-time observation of MB delivery to a region of interest, such as tumor regions, and a US destruction pulse can destroy the MBs to release the drug.10 Moreover, exposure of MBs to US may result in a transient increase in tumor permeability. Therefore, US-induced MB destruction can be used for the treatment of tumors by taking advantage of high local drug concentrations and transiently increased capillary permeability.11,12 However, MBs has poor drug-loading efficiency (LE%) because of their thin shells and the volume of perfluorocarbon gas they carry. Effective blood concentrations of numerous anticancer drugs cannot be reached when MBs are used as carriers; this disadvantage greatly limits the application of MBs as drug delivery vehicles.
In contrast to MBs, nanoparticles do not respond to US and can efficiently carry drugs. Meanwhile, albumin is considered a desirable drug-loading carrier for fabricating nanoparticles because of its nontoxic, non-antigenic, biocompatible, and biodegradable properties, as well as its ability to bind with various drugs.13,14 Albumin is also favored as a raw material for drug delivery owing to its abundance and physical and chemical robustness.14–16 Albumin nanoparticles can encapsulate both hydrophilic and hydrophobic drugs.17 After being encapsulated in the albumin nanoparticles, hydrophobic drugs such as HK could be well dispersed in aqueous solutions in the form of suspension. In this way, the requirements of administration are met.
We took advantage of the acoustic properties of MBs and the high loading capacity of nanoparticles to create a new drug delivery system (DDS) by encapsulating nanoparticles into MBs. This new DDS possesses the drug-loading capacity of nanoparticles and can still deliver drugs specifically to target tissues when exposed to US. These advantages provide enhanced drug delivery to tumor tissues while minimizing the harmful systemic side effects of the anticancer drugs.
In this study, a novel DDS in which HK-loaded albumin nanoparticles (HKNs) were encapsulated MBs, was employed to treat Lewis lung carcinoma (LLC) in solid tumor model. The morphology, LE%, and encapsulation efficiency (EE%) of the prepared HKN-loaded MBs (HKN-MBs) were characterized, and the in vivo anticancer effect of HKN-MBs exposed to US was estimated using the LLC tumor-bearing C57BL/6 mice.
Experimental
Materials and animals
Honokiol (HK) (purity ≥98%) was purchased from Xi'an Grass Plant Science and Technology (Shanxi, China). Bovine serum albumin (BSA) was obtained from Roche (Switzerland). Lecithin was gained from Shuangxuan Microbe Culture Medium Products Factory (Peking, China). Fluorescein isothiocyanate (FITC, purity ≥90%) was purchased from Aladdin (Shanghai, China). Sephadex G-50 was obtained from Pharmacia (USA). All chemicals used in this study were of analytical grade and used without further processing. Perfluorocarbon gas was supplied from Institute of Ultrasound Imaging of Chongqing Medical University (Chongqing, China). The LLC cells were provided by Chongqing Key Laboratory of Biochemistry and Molecular Pharmacology (Chongqing, China). C57BL/6 mice (four-week-old, 18–22 g) were supplied from the Laboratory Animal Center in Chongqing Medical University (Chongqing, China). Animal care and work protocols were approved by the institutional Animal Care and Use Committee of Chongqing Medical University (Chongqing, China).Preparation of HKNs
HKNs were prepared using a modified desolvation technique as previously described.18–20 Briefly, 40 mg of BSA was dissolved in 4.0 mL of 0.01 mM phosphate-buffered solution (PBS, pH 7.4). Under constant stirring, desolvation of 1% BSA solution was obtained by dropwise addition of 24.0 mL HK–ethanol solution (0.067%, w/v). Subsequently, 50 μL of glutaraldehyde (0.5%) aqueous solution was added to the mixture to induce particle cross-linking by stirring for 4 h. Organic solvents were removed through rotary vacuum evaporation at 35.0 °C. The nanoparticle suspension was then diluted to 5.0 mL for further use.Preparation of HKN-MBs
HKN-MBs were prepared using a mechanical shaking method.21,22 Briefly, 25.2 mg of lecithin, 243.6 mg of glycerin, and 300 μL of HKNs were added to 0.42 mL of PBS (81.0 mM NaCl, 16.8 mM Na2HPO4, and 3.2 mM KH2PO4) in 4 mL vials. Perfluorocarbon gas was then injected in the vial to expel air. The vial was swirled to mix the components and incubated at 50 °C for 2 h. Using a vortex mixer (2800 rpm, XW-80A, Shanghai, China), the vial was swirled for 1 min and centrifuged at 2000 rpm for 5 min (Thermo Scientific Heraeus Fresco 21 centrifuge, USA). The upper layer was taken out and cooled to 20 °C. The prepared HKN-MBs were then stored at 4 °C for further studies.Blank MBs (i.e., without nanoparticles) were similarly prepared using the method mentioned above but without the addition of HKNs.
Characterization of HKN-MBs
The mean diameter and size distribution of the blank MBs and HKN-MBs were determined using a Malvern Zetasizer Nano ZS instrument (Malvern Instrument, UK). The morphologies of blank MBs and HKN-MBs were observed using an optical microscope (Nikon ECLIPSE 50i, Japan).To further confirm the structure of the HKN-MBs, FITC-labeled BSA were prepared and used in the preparation of HKNs.19 Then, the FITC-labeled HKN-MBs were observed using confocal laser scanning microscope (CLSM, Nikon A1R, Japan).
Entrapment efficiency & HK loading
HKN-MBs were dissolved in pure methanol, and the HK encapsulated in the MBs was measured at a wavelength of 292 nm using RP-HPLC (Agilent 1260 HPLC system, USA). A LiChrospher RP C18 column (4.6 mm × 150 mm, 5 μm, Hanbon Sci. & Tech., China) was used, and the mobile phase was a mixture of methanol and water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v). The flow rate was 1.0 mL min−1. All tests were performed at a wavelength of 292 nm with a constant temperature of 30 °C.23 Experiments were conducted in triplicate. EE% and LE% were calculated by the following equations,24 respectively:
20, v/v). The flow rate was 1.0 mL min−1. All tests were performed at a wavelength of 292 nm with a constant temperature of 30 °C.23 Experiments were conducted in triplicate. EE% and LE% were calculated by the following equations,24 respectively:| EE (%) = WHK/Wtot × 100% | (1) |
| LE (%) = WHK/WBL × 100% | (2) |
US imaging of HKN-MBs in agarose gel phantom
US contrast imaging was conducted on a Doppler ultrasonic diagnostic apparatus25 (Mylab90, Esaote, Italy). Normal saline, HKNs, blank MBs, and HKN-MBs were placed in 2% agarose gel phantom, where the probe was placed on one side. The results of US imaging pictures were saved for later use.Cell lines and culture
The LLC cells were supplied by the Chongqing Key Laboratory of Biochemistry and Molecular Pharmacology. The cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution at 37 °C in a fully humidified incubator maintained with 5% CO2. For in vivo injection, LLC cells in logarithmic growth phase were trypsinized and centrifuged at 1000 rpm for 5 min, washed twice, and reconstituted in serum-free DMEM medium at a concentration of 2 × 107 cells per mL for 0.2 mL subcutaneous injections.Animal model
The mice were bred at a constant temperature and humidity. Murine LLC cells were used to create a solid tumor model. Under aseptic conditions, LLC cells (4 × 106 cells per 0.2 mL per mouse) were suspended in serum-free DMEM medium and administered subcutaneously to the dorsal flanks of the mice. In vivo tumor imaging and antitumor effect experiments were performed 10 to 15 days after tumor inoculation when the volumes of tumor tissues reached about 0.5 cm3. These cancer xenograft C57BL/6 mice were used for in vivo tumor imaging and antitumor effect experiments. All procedures including the use and care of mice were approved ethically and scientifically by the Animal Ethical Commission of Chongqing Medical University in compliance with the Practice Guidelines for Laboratory Animals of China.In vivo tumor imaging
To evaluate the contrast enhancement of HKN-MBs at tumor site, US imaging was performed using a Doppler ultrasonic diagnostic apparatus (Mylab90, Esaote, Italy). LLC-tumor-bearing mice received 0.2 mL injections of HKN-MBs through the tail vein. Tumors were then monitored with contrast-enhanced ultrasonography and pictures were captured. US imaging pictures of blank MBs, HKNs, and saline were also acquired.In vivo antitumor effect
The transplanted LLC-tumor-bearing mice were randomly divided into four groups (five mice per group). For six consecutive days, the experimental groups were injected with the same volume (0.2 mL) of HKNs, HKN-MBs, and HKN-MBs with US exposure (HKN-MB + US) once a day; the dose of HK in all groups was 20 mg kg−1. The control group was treated with saline. Each mouse received 0.2 mL bolus injection through the tail vein. In the HKN-MB + US group, a handheld probe (Model UTG 1025, Institute of US Imaging of Chongqing Medical University, China) was placed in the tumor site immediately after injecting with HKN-MBs. The instrument was intermittently operated for 30 s and turned off for another 30 s continuously within 6 min, and the US power intensity was 2 W cm−2. Over the next seven days, the tumors were measured daily with a Vernier caliper and the volume (V) of the tumor tissues were calculated using eqn (3).| V (mm3) = (A × B2)/2 | (3) |
After the treatments were completed, the mice were sacrificed and the tumors were harvested. The masses of tumors were weighed and the rate of tumor growth inhibition (RTGI) was calculated using the following eqn (4):
| RTGI% = [(average tumor weight of control group − average tumor masses of tested group)/average tumor weight of control group] × 100% | (4) |
Histology observation
The tumor tissues of the mice which have been treated with saline, HKNs, HKN-MBs, and HKN-MB + US, were collected for histology observation. The tumors were excised from the mice and fixed in 10% neutral buffered formalin. Thereafter, the tissues were embedded routinely in paraffin, sectioned at a thickness of 4 μm, and stained with hematoxylin (H) and eosin (E). Then, the slices obtained were examined by optical microscopy.Statistical analysis
All statistical tests were performed using the statistical software package SPSS 18.0. The p-value of 0.05 was used as the significance level for all tests. An analysis of variance test was performed on the data. All data were reported as mean ± standard deviation.Results
Characterization of blank MBs and HKN-MBs
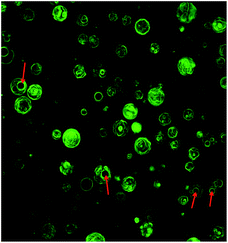
Fig. 1 shows that after centrifugation at 2000 rpm for 5 min, both blank MBs and HKN-MBs rose to the upper layer. However, they exhibited homogeneous turbidity when gently shaken. This behavior may have resulted from the decrease in the density of HKN-MBs and blank MBs after perfluorocarbon gas was encapsulated in the MBs. To further investigate the internal composition of the HKN-MBs, the FITC-labeled HKN-MBs were observed using CLSM. As shown in Fig. 2, the green fluorescence represented the HKNs, and the red arrows pointed to the encapsulated fluorocarbon gas. | ||
| Fig. 1 The appearance of blank MBs after centrifugation (A), blank MB after shaking (B), HKN-MBs after centrifugation (C), and HKN-MBs after shaking (D). | ||
The loading amount of HK in MBs was determined using HPLC after the total amount of drugs was extracted from HKN-MBs. The EE% and LE% of HKN-MBs were 67.78% ± 1.38% and 3.48% ± 0.20%, respectively. To verify whether combining MBs and nanoparticles could improve drug-loading capacity, we replaced HKNs with HK to prepare traditional HK-loaded MBs using the method mentioned above. The results showed that the LE% of traditional MBs was 0.022%, which was significantly less than that of HKN-MBs.
Particle size distribution
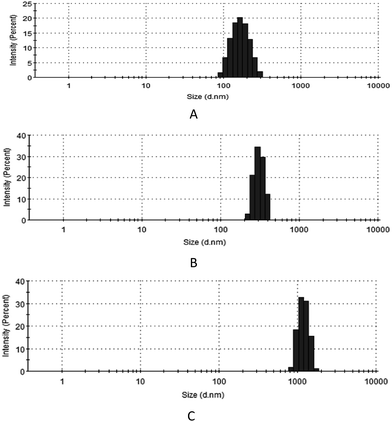
Fig. 3 shows that the mean diameters of HKNs, blank MBs and HKN-MBs were 159.3 ± 45.03, 470.5 ± 46.21 and 1447.0 ± 182.4 nm, respectively. These results indicate that HKNs loading increased the size of the MBs. During the preparation process, MBs were formed through the self-assembly of phospholipids when perfluorocarbon gas was introduced and mixed in a vortex mixer. More nanoparticle suspensions were encapsulated by MBs because the viscosity of the nanoparticle suspension was higher than that of PBS solution, which increased the volume of MBs.Optical micrographs of HKNs, blank MBs, and HKN-MBs
Fig. 4A shows the light microscopic images of HKNs, blank MBs, and HKN-MBs at 100× magnification under an optical microscope (Nikon ECLIPSE 50i, Japan).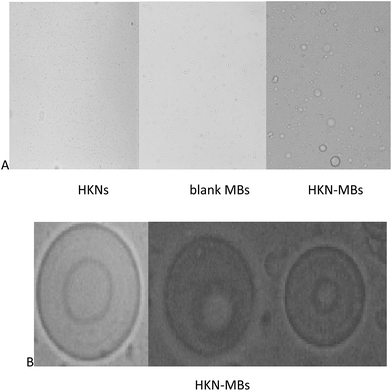 | ||
| Fig. 4 (A) Images of HKNs (×100), blank MBs (×100), and HKN-MBs (×100) under the optical microscope; (B) images of HKN-MBs (×400) under the optical microscope. | ||
HKNs are difficult to identify under the light microscope because of its small size. HKN-MBs are evidently larger than blank MBs, which indicated that HKN loading increased the size of the MBs. These results are consistent with the findings of the particle size distribution analysis.
The internal composition of HKN-MBs was further observed under 400× magnification. As shown in Fig. 4B, a round bubble can be seen in the phospholipid membrane of HKN-MBs.
In vitro analysis of US-enhanced imaging
Fig. 5A shows the US images of saline, HKNs, blank MBs, and HKN-MBs. Both saline and HKNs showed no US contrast imaging effects. However, both HKN-MBs and blank MBs brought about an enhanced US imaging in 2% agarose gel phantom. Furthermore, no significant difference was shown between the images of HKN-MBs and those of blank MBs. | ||
| Fig. 5 (A) In vitro US images of saline, HKNs, blank MBs and HKN-MBs; (B) US images of one min after intravenous injection of saline, HKNs, blank MBs, and HKN-MBs at tumor. | ||
In vivo analysis of US-enhanced imaging
To evaluate the US imaging effect of HKN-MBs in vivo, US imaging experiments on saline, HKNs, blank MBs, and HKN-MBs in LLC-tumor-bearing mice were conducted, and results are shown in Fig. 5B. The HKNs and saline groups showed no US images, whereas both HKN-MBs and blank MBs improved US imaging on the tumor sites. Moreover, no significant difference in US images was found between HKN-MBs and blank MBs.In vivo antitumor activity
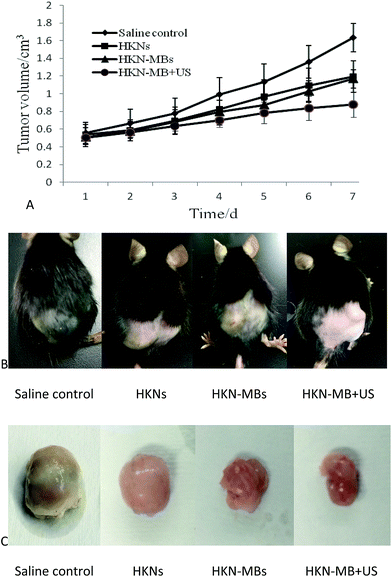
Five days after the inoculation of LLC cells, palpable tumor nodules emerged on the dorsal flank area of all mice. The lumps were irregularly shaped and infiltrated the skin without ulceration or hemorrhage on the surface. According to the tumor growth curve in Fig. 6A, the mice in the HKN-MB + US group exhibited the slowest tumor growth rate. The tumor growth rates of mice treated with HKNs and HKN-MBs were relatively slower than those treated with saline control, and no significant difference was observed between the two groups.The tumor weight and inhibition rates in each group are displayed in Table 1. The mean tumor weights of mice treated with HKNs, HKN-MBs, and HKN-MB + US were all significantly lower than those treated with saline. The maximum tumor inhibition rate was found in the HKN-MB + US group. We also found that the growth of LLC tumor treated with HKNs and HKN-MBs was suppressed to a certain extent compared with those in the saline control group.
Photographs of tumor-bearing mice and the excised tumors from each group are presented in Fig. 6B and C, respectively.
Next, we observed pathological sections of the tumors. As shown in Fig. 7, varying degrees of necrosis were observed in the tumors of experimental groups compared with those in the saline control group. The tumor cells from the mice that received saline in the control group were tightly arranged. Numerous living cells with large nuclei and apparent nucleoli were observed. However, the tumor cells in the groups of HKNs and HKN-MBs were arranged loosely, and a relatively small-scale of necrotic region was observed. Moreover, large areas of necrotic regions in the tumors were observed from the HKN-MB + US group. Moreover, the tumor cells were fragmented or absent cell bodies. From the above, the HKN-MB + US group exhibited more necrosis regions in contrast to the other groups. These results further confirmed the anticancer efficacy of HKN-MB + US.
Discussion
The technology of US-induced MB destruction is used for drug and gene delivery and has the advantages of being controllable and efficient without the risk of receptor saturation. Traditional MB has very low drug-loading capacity because its thin phospholipid layer cannot accommodate enough drugs. Only a small amount of drugs, which have very low effective blood concentration, can be used as candidate drugs for the MB carriers. The MBs prepared in this study encapsulate the drug-loaded nanoparticles and gas simultaneously, which indicated that the drug-loading capacity of MB was improved by the high loading capacity of nanoparticles. Thus, the MBs prepared in this study could be used with other antitumor drugs. The prepared HKN-MBs displayed a LE% of 3.48% ± 0.20%, which is significantly higher than that of traditional HK-loaded MBs.The results of US imaging experiments showed that the MBs still possessed the characteristics of acoustic response after encapsulating the nanoparticles. When the nanoparticles were encapsulated by the MBs, the loss of nanoparticle was reduced before the target region was reached. The nanoparticle-loaded MBs were injected into the vasculature and fragmented when exposed to the US destruction pulse. The antitumor drug-loaded nanoparticles were released at the target region and accumulated within the tumor interstitium as a result of the EPR effect. Moreover, US transiently changed the permeability of the cells' membrane, which improved the cellular uptake of nanoparticles.
Conclusions
HKNs and perfluorocarbon gas were successfully encapsulated in MBs. The prepared HKN-MBs displayed a mean diameter of 1447.0 ± 182.4 nm, as well as EE% and LE% of 67.78% ± 1.38% and 3.48% ± 0.20%, respectively. Both in vitro and in vivo US imaging experiments showed that HKN-MBs significantly improved US imaging compared with saline and HKNs. Thus, HKN-MBs possess good acoustic properties. In in vivo antitumor experiments, tumor-bearing mice were administered with HKN-MBs through the tail vein and were exposed to US, which increased the rate of tumor growth inhibition compared with those of HKN-MBs and HKN treatments. Therefore, encapsulation of albumin nanoparticles in lecithin MBs may provide a feasible technique for targeted tumor drug delivery in clinical therapy.Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.Acknowledgements
This project was supported by the National Research Foundation for the Doctoral Program of Higher Education of China (No. 20125503120003), Natural Science Foundation Project of CQ CSTC (No. cstc2012jjA10021), and Students' Research and Innovation Experimental Project of Chongqing Medical University (201432, 201244).Notes and references
- Q. Q. Jiang, L. Y. Fan, G. L. Yang, W. H. Guo, W. L. Hou, L. J. Chen and Y. Q. Wei, BMC Cancer, 2008, 8, 242 CrossRef PubMed.
- F. Fang, C. Gong, Z. Qian, X. Zhang, M. Gou, C. You, L. Zhou, J. Liu, Y. Zhang, G. Guo, Y. Gu, F. Luo, L. Chen, X. Zhao and Y. Wei, ACS Nano, 2009, 3, 4080–4088 CrossRef CAS PubMed.
- P. Steinmann, D. K. Walters, M. J. Arlt, I. J. Banke, U. Ziegler, B. Langsam, J. Arbiser, R. Muff, W. Born and B. Fuchs, Cancer, 2012, 118, 2117–2127 CrossRef CAS PubMed.
- P. A. Dijkmans, L. J. Juffermans, R. J. Musters, A. van Wamel, F. J. ten Cate, W. van Gilst, C. A. Visser, N. de Jong and O. Kamp, Eur. J. Echocardiogr., 2004, 5, 245–256 CrossRef CAS PubMed.
- F. Kiessling, S. Fokong, P. Koczera, W. Lederle and T. Lammers, J. Nucl. Med., 2012, 53, 345–348 CrossRef CAS PubMed.
- X. Wang, P. Liu, W. Yang, L. Li, P. Li, Z. Liu, Z. Zhuo and Y. Gao, Int. J. Nanomed., 2014, 9, 4899–4909 Search PubMed.
- J. J. Kwan and M. A. Borden, Adv. Colloid Interface Sci., 2012, 183–184, 82–99 CrossRef CAS PubMed.
- R. Bekeredjian, H. F. Kuecherer, R. D. Kroll, H. A. Katus and S. E. Hardt, Urology, 2007, 69, 386–389 CrossRef PubMed.
- S. Wu, L. Li, G. Wang, W. Shen, Y. Xu, Z. Liu, Z. Zhuo, H. Xia, Y. Gao and K. Tan, Int. J. Nanomed., 2014, 9, 5639–5651 Search PubMed.
- S. A. Peyman, R. H. Abou-Saleh and S. D. Evans, Ther. Delivery, 2013, 4, 539–542 CrossRef CAS PubMed.
- R. Bekeredjian, R. D. Kroll, E. Fein, S. Tinkov, C. Coester, G. Winter, H. A. Katus and H. Kulaksiz, Ultrasound Med. Biol., 2007, 33, 1592–1598 CrossRef PubMed.
- L. Shi, P. Palacio-Mancheno, J. Badami, W. Shin da, M. Zeng, L. Cardoso, R. Tu and B. M. Fu, Int. J. Nanomed., 2014, 9, 4437–4448 Search PubMed.
- T. Peters Jr, Adv. Protein Chem., 1985, 37, 161–245 CrossRef CAS.
- F. F. An, Y. L. Yang, J. Liu, J. Ye, J. F. Zhang, M. J. Zhou, X. J. Zhang, C. J. Zheng, X. J. Liang and X. H. Zhang, RSC Adv., 2014, 4, 6120–6126 RSC.
- Z. Yang, W. Gong, Z. Wang, B. Li, M. Li, X. Xie, H. Zhang, Y. Yang, Z. Li, Y. Li, F. Yu and X. Mei, Int. J. Pharm., 2015, 490, 412–428 CrossRef CAS PubMed.
- L. Jiang, X. Zhao, C. Zheng, F. Li, J. L. Maclean, F. Chen, A. Swami, H. Qian, J. Zhu and L. Ge, RSC Adv., 2015, 5, 34956–34966 RSC.
- T. E. Stinchcombe, Nanomedicine, 2007, 2, 415–423 CrossRef CAS PubMed.
- S. J. Mao, S. X. Hou, R. He, L. K. Zhang, D. P. Wei, Y. Q. Bi and H. Jin, World J. Gastroenterol., 2005, 11, 3075–3079 CrossRef CAS PubMed.
- L. Zhang, S. Hou, S. Mao, D. Wei, X. Song and Y. Lu, Int. J. Pharm., 2004, 287, 155–162 CrossRef CAS PubMed.
- L. K. Zhang, S. X. Hou, J. Q. Zhang, W. J. Hu and C. Y. Wang, Arch. Pharmacal Res., 2010, 33, 1193–1198 CrossRef CAS PubMed.
- S. Zhou, S. Li, Z. Liu, Y. Tang, Z. Wang, J. Gong and C. Liu, J. Exp. Clin. Cancer Res., 2010, 29, 170 CrossRef CAS PubMed.
- J. Kang, X. Wu, Z. Wang, H. Ran, C. Xu, J. Wu and Y. Zhang, J. Ultrasound Med., 2010, 29, 61–70 Search PubMed.
- C. Xu, Y. Tang, W. Hu, R. Tian, Y. Jia, P. Deng and L. Zhang, Carbohydr. Polym., 2014, 113, 9–15 CrossRef CAS PubMed.
- Y. Zheng, Y. Zhang, M. Ao, P. Zhang, H. Zhang, P. Li, L. Qing, Z. Wang and H. Ran, J. Microencapsulation, 2012, 29, 437–444 CrossRef CAS PubMed.
- W. T. Chen, S. T. Kang, J. L. Lin, C. H. Wang, R. C. Chen and C. K. Yeh, Biomaterials, 2015, 53, 699–708 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |