Synthesis, biological evaluation and docking studies of some novel isatin-3-hydrazonothiazolines†
Abstract
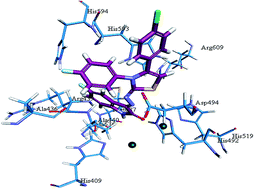
A new series of thirty nine 5-trifluoromethoxy/fluoro/chloro-isatin 3-hydrazonothiazolines 5a–n, 6a–o and 7a–j were synthesized by cyclization of the corresponding intermediate N4-aryl-substituted isatin-3-thiosemicarbazones 3 (prepared by condensation of appropriate isatin 1 with appropriate N4-aryl-substituted 3-thiosemicarbazides 2) with 4-chlorophenacyl bromide 4 in absolute ethanol or ethanol–benzene mixture and screened for their cytotoxicity, phytotoxicity, antifungal and urease inhibitory potential. All the synthesized compounds were found to be almost inactive in a brine shrimp (Artemia salina) bioassay, demonstrating IC50 values > 1.62 × 10−4 to 2.17 × 10−4 M. In a phytotoxicity assay, out of thirty-nine compounds tested, six i.e. 5i, 6h, 6i, 6k, 7c and 7h proved to be active, showing weak or non-significant (5–30%) activity at the highest tested concentration (500 μg mL−1). Similarly, in antifungal assay, twenty-six compounds i.e. 5a, 5b, 5d–f, 5h–j, 5m, 6a, 6b, 6d, 6j, 6l–o, 7a, 7b and 7d–j were found to be active against one, two, or three selected fungal strains, exhibiting weak or non-significant inhibition (10–30%). Of these, 6d and 6o displayed a relatively better activity profile in terms of the number of organisms inhibited. On the other hand, in a urease inhibition bioassay, all the synthesized hydrazonothiazolines proved to be potent enzyme inhibitors, demonstrating inhibitory activity with IC50 values ranging from 3.70 ± 0.62 to 849 ± 2.26 μM. Compounds 5c, 5g–i, 5k, 5n, 6b, 6c, 6i, 6k, 6l, 6n, 6o, 7a, 7e, 7i and 7j were, however, found to be relatively very potent, displaying outstanding enzymatic activity (IC50 = 3.70 ± 0.62 to 20.9 ± 0.57 μM), even better than the reference inhibitor thiourea (IC50 = 22.3 ± 1.12 μM), and may thus act as valid leads for further studies. Molecular docking studies of the synthesized isatin–thiazolines 5a–n, 6a–o and 7a–j were also carried out to elucidate their relationship with the binding pockets of the enzyme. This study offers the first example of exhibition of urease inhibitory potential by isatin–thiazolines and as such provides a solid basis for further research on these compounds to develop more potent antiurease compounds of medicinal/agricultural interest.

 Please wait while we load your content...
Please wait while we load your content...