Observation of exceptional ‘de Vries-like’ properties in a conventional aroylhydrazone based liquid crystal†
Hemant Kumar Singh‡
a,
Sachin Kumar Singh‡a,
Rajib Nandib,
D. S. Shankar Raoc,
S. Krishna Prasadc,
Ranjan K. Singhb and
Bachcha Singh*a
aDepartment of Chemistry (Centre of Advance Study), Institute of Science, Banaras Hindu University, Varanasi-221005, India. E-mail: bsinghbhu@rediffmail.com
bDepartment of Physics (Centre of Advance Study), Institute of Science, Banaras Hindu University, Varanasi-221005, India
cCentre for Nano and Soft Matter Sciences, Jalahalli, Bangalore-560013, India
First published on 25th May 2016
Abstract
Smectic liquid crystals with ‘de Vries-like’ properties are characterized by a maximum layer contraction of ≤1% upon transition from the non-tilted SmA phase to the tilted SmC phase. A straight alkoxy chain-terminated conventional aroylhydrazone based liquid crystal N-(4-(tetradecyloxy)-2-hydroxybenzylidene)-4′((4′′(hexadecyloxy)benzyl)oxy)benzohydrazide (BSac-1416) undergoes the SmA–SmC phase transition with a maximum layer contraction of 0.65%. The merits of the de Vries reduction factor, i.e. R and f values for this mesogen, are comparable to, or even lower than, those reported for established perfluorinated and polysiloxane-terminated bona fide de Vries-like materials.
Smectic A (SmA) and smectic C (SmC) liquid crystals are layered phases possessing quasi-long-range positional order in one dimension represented by a mass-density wave.1 The wave vector of this density wave is along the director in the case of the SmA phase, while it is tilted in the case of the SmC phase. Owing to this feature, the layer spacing is significantly lower in the tilted SmC phase than in the orthogonal SmA phase.2 Maximum layer-shrinkage in a typical SmA–SmC transition can be as high as 11%.3,4
In 1972, Diele et al. reported a material in which the layer spacing was similar in the SmA and SmC phases.5 To explain this feature, de Vries suggested a new type of SmA phase in which the molecules are tilted with respect to the layer normal just as in the SmC phase but the tilt directions of different layers are uncorrelated.6 These are now known as the de Vries smectic phases and are characterized by a maximum layer contraction of ≤1% upon transition from the non-tilted SmA phase to the tilted SmC phase.4,7–15 This phenomenon stems from the diffuse cone model proposed by de Vries that describes the SmA phase as a lamellar structure in which mesogens have a tilted molecular orientation and random azimuthal distribution.16 According to this idealized model, the SmA–SmC transition is described as an ordering of the azimuthal distribution that results in zero layer contraction, as shown in Fig. 1. The simple reorientation of molecules at the SmA–SmC transition in de Vries-like liquid crystals is advantageous for practical applications. For instance, the low layer contraction avoids the formation of a chevron structure and zigzag defects that usually occur in SmA–SmC transitions of conventional ferroelectric (SmC*) liquid crystals and that are detrimental for the optical quality of electro-optical devices.17 High electroclinic effects (response, tilt angle, and birefringence) can be achieved at low voltages as compared with typical electroclinic materials on these materials.18–20
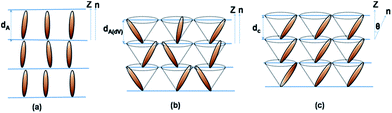 | ||
| Fig. 1 Schematic representation of the molecular arrangement in (a) SmA (b) one type of SmAdV and (c) SmC phases. The molecules are along the layer normal in the SmA, while being tilted in the SmAdV and SmC phases. To be noted is the fact that the tilt is azimuthally correlated in the SmC but not in the SmAdV phase (dV: de Vries).21 | ||
The structure of this unusual phase has yet to be fully elucidated, but recent theoretical studies suggest that materials combining low orientational order and high lamellar order are likely to exhibit this behavior.22,23 Three examples of liquid crystal materials known to have very low layer shrinkage and large electroclinic susceptibilities are considered as bona fide ‘de Vries-like’ liquid crystals. These are the siloxane-terminated mesogen TSiKN65 and its carbosilane-terminated analogue W599,9,24 and the 2-phenylpyrimidine mesogen 3M 8422 with a chiral perfluoroether chain10 which show maximum layer contractions in the rage of 0.40–0.73% (Fig. 2). In our earlier reports of X-ray, electrical, electro-optical and dielectric studies on such bona fide de Vries LCs, the effect of differential interactions in different molecular end of compounds was found to be one of the major cause of unusual behaviour and minimum layer shrinkage in such systems.25,26 Rational design strategy and structural investigations of de Vries-like liquid crystals was initiated by Lemieux et al. by design and synthesis of a number of de Vries-like liquid crystals having trisiloxane terminated side-chain materials with 2-phenylpyrimidine core.27–29 Further, they extended their work by incorporating chemically inert carbosilane-terminated de Vries LCs and substituting the 2-phenylpyrimidine core with the cores known to be stronger ‘SmC-promoters’ in model compounds: 6-phenylpyridazine, 6-phenylpyridine and 2-phenyl thiadiazole.30,31 In the same reports, drawback of hydrolytical and electrochemical lability of siloxane end group and need of incorporation of chemically inert nanosegregating terminal elements for fabrication of de Vries-like LCs towards electro-optical applications are mentioned.27,28 Thus, reports on bona fide de Vries-like materials feature nanosegregating structural elements such as siloxane end-groups or perfluorinated side chains and example of simple alkoxy chain-terminated conventional liquid crystal showing de Vries-like properties is still rare. In this communication, we report a structurally unprecedented achiral, chemically inert, straight alkoxy chain-terminated aroylhydrazone based liquid crystal N-(4(tetradecyloxy)-2-hydroxybenzylidene)-4′((4′′(hexadecyloxy)benzyl)oxy)benzohydrazide (BSac-1416) showing de Vries like properties comparable to that of perfluorinated or siloxane end-groups containing bona fide de Vries-like liquid crystalline materials.
 | ||
| Fig. 2 Molecular structures of bona fide de Vries-like liquid crystals (TSiKN65, W599, 3M 8422, 2)8,9,18 and BSac-1416. | ||
Compound BSac-1416 and initial precursors were obtained by microwave assisted synthetic approach in very good yield (Scheme 1). The detailed experimental procedures and analytical data are described in the ESI.†
The XRD results, microscopic optical observations and differential scanning calorimetry (DSC) enthalpies at the phase transitions have been used to determine mesomorphic properties and de Vries-like behaviour in the newly synthesized compound BSac-1416. Accurate measurements of the smectic layer spacing (d) as a function of temperature were carried out by SAXS.
As shown in Fig. 3, the normalized d/dAC(T) profiles of BSac-1416 shows a negative thermal expansion in the SmA phase that persists in the SmC phase and counteracts the layer contraction caused by tilting in the SmC phase. Other bona fide ‘de Vries-like’ materials have been reported to show similar negative thermal expansion in their d(T) profiles.8,13–15,25 Maximum layer contractions of 0.65% are observed at ca. 10 K below TAC and the layer spacing at the SmA–SmC transition dAC is restored upon further cooling to ca. 26.4 K below TAC.
The compound BSac-1416 forms SmA and SmC phases. A thin film (18 μm thickness) of the sample observed between crossed polarizers, exhibits the characteristic fan and homeotropic textures of the SmA phase that turn into broken fan texture, upon transition to the SmC phase. In each case, the texture analysis revealed a pronounced change in interference colour on cooling from the isotropic phase to the SmC phase. As shown in Fig. 4, the fan texture shows a green colour just below the clearing point, which gradually turns reddish-green at the SmA–SmC transition point TAC, and then red at 6 °C below TAC. The overall colour change corresponds to a net increase in birefringence Δn from ca. 0.07 to 0.08 consistent with an increase in orientational order expected for de Vries-like materials.9
The optical tilt angle (θopt) was measured by polarized optical microscopy (POM) as a function of reduced temperature T − TAC in the absence of electric field (Fig. 5); θopt, obtained as the difference in the angle of rotation between dark states in domains of opposite tilt orientations30 was found to be 26.1°. Its magnitude shows a discontinuity as T approaches TAC suggesting that the SmA–SmC transition may be weakly first-order in nature. To test this hypothesis, the θopt profile was fitted to the power law:
| θ ∝ |T − Tc|β | (1) |
 | ||
| Fig. 5 Optical tilt angle θopt versus reduced temperature T − TAC for the compound BSac-1416. The solid lines represent the fits to eqn (1) (β = 0.21). | ||
To assess the potential of a de Vries material to achieve defect-free bookshelf geometry in the SmC phase, Radcliffe et al. defined figures of merit R and f according to eqn (2) and (3):
| R = δ(T)/θopt(T) = cos−1[dC(T)/d(TAC)]/θopt(T) | (2) |
| f = θXray(T)/θopt(T) = cos−1[dC(T)/dA(T)]/θopt(T) | (3) |
Here, assuming a rigid-rod model δ(T) is the tilt angle required to give the observed contraction relative to the layer spacing at the SmA–SmC transition.8 In eqn (3), θXray(T), the X-ray tilt required to give the observed layer contraction relative to the SmA layer spacing dA(T) extrapolated in the SmC phase. This extrapolation is done at a given reduced temperature using least-squares fit of the data points in the SmA phase near the SmA–SmC transition.10 The latter gives a reduction factor that is normalized with respect to negative thermal expansion. In each equation, the tilt angle derived from layer spacing measurements is divided by the optical tilt angle θopt(T) measured by POM. At a reduced temperature T − TAC = −10 K, θXray observed for BSac-1416, is 6.1°, a value consistent with the data for other “de Vries like” materials.10 The R and f values of this compound at T − TAC = −10 K were calculated to be 0.23 and 0.44 respectively. Table 1 shows R and f values for the title compound under investigation along with other “de Vries-like” liquid crystals. It is seen that the figures of merit for BSac-1416 are comparable to those for the best “de Vries-like” materials reported heretofore, and thus has the potential to achieve defect free bookshelf geometry.4
In summary, “de Vries-like” liquid crystals behaviour comparable to earlier best reports on such liquid crystals has been observed in a simple straight alkoxy chain-terminated conventional liquid crystal. Ongoing efforts are focused on structure–function relationship studies of homologous series of such mesogens featuring aroylhydrazone core and reduction in melting point of mesogens via structural modifications, and will be reported in due course.
Acknowledgements
HKS (Senior Research Fellow) is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for financial support. BS is grateful to DRDO, India (Project No. ERIP/ER/1203053/M/01/1542) and DST, India (Project No. SR/S1/IC-08/2012) for providing financial assistance. S.K.S. is grateful to DST, SERB for providing fellowship under N-PDF (PDF/2015/000147) scheme.Notes and references
- S. Chandrasekhar, Liquid Crystals, Cambridge University Press, Cambridge, England, 2nd edn, 1992 Search PubMed.
- C. V. Lobo, S. K. Prasad and D. S. Shankar Rao, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 72, 62701 CrossRef PubMed.
- S. Kumar, Phys. Rev. A: At., Mol., Opt. Phys., 1981, 23, 3207 CrossRef CAS.
- J. P. Lagerwall and F. Giesselmann, ChemPhysChem, 2006, 7, 20 CrossRef CAS PubMed.
- S. Diele, P. Brand and H. Sackman, Mol. Cryst. Liq. Cryst., 1972, 16, 105 CrossRef CAS.
- (a) A. De Vries, in Abstracts of the Fifth International Liquid Crystal Conference, Stockholm, ed. G. H. Brown, ILC Society, Kent, OH, 1974, p. 150 Search PubMed; (b) A. De Vries, Mol. Cryst. Liq. Cryst., 1977, 41, 27 CrossRef CAS; (c) A. De Vries, A. Ekachai and N. Spielberg, Mol. Cryst. Liq. Cryst., 1979, 49, 143 CrossRef CAS.
- Y. Takanishi, Y. Ouchi, H. Takezoe, A. Fukuda, A. Mochizuki and M. Nakatsuka, Jpn. J. Appl. Phys., 1990, 2, L984 CrossRef.
- M. D. Radcliffe, M. L. Brostrom, K. A. Epstein, A. G. Rappaport, B. N. Thomas, R. Shao and N. A. Clark, Liq. Cryst., 1999, 26, 789 CrossRef CAS.
- M. S. Spector, P. A. Heiney, J. Naciri, B. T. Weslowski, D. B. Holt and R. Shashidhar, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 61, 1579 CrossRef CAS.
- J. P. F. Lagerwall, F. Giesselmann and M. D. Radcliffe, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2002, 66, 31703 CrossRef PubMed.
- J. Naciri, C. Carboni and A. K. George, Liq. Cryst., 2003, 30, 219 CrossRef CAS.
- O. E. Panarina, Y. P. Panarin, J. K. Vij, M. S. Spector and R. Shashidhar, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 67, 51709 CrossRef CAS PubMed.
- M. Rossle, R. Zentel, J. P. F. Lagerwall and F. Giesselmann, Liq. Cryst., 2004, 31, 883 CrossRef.
- N. Hayashi, T. Kato, A. Fukuda, J. K. Vij, Y. P. Panarin, J. Naciri, R. Shashidhar, S. Kawada and S. Kondoh, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 71, 41705 CrossRef PubMed.
- O. E. Panarina, Y. P. Panarin, F. Antonelli, J. K. Vij, M. Reihmann and G. Galli, J. Mater. Chem., 2006, 16, 842 RSC.
- F. Giesselmann, P. Zugenmaier, I. Dierking, S. T. Lagerwall, B. Stebler, M. Kaspar, V. Hamplova and M. Glogarova, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1999, 60, 598 CrossRef CAS.
- A. de Vries, J. Chem. Phys., 1979, 71, 25 CrossRef CAS.
- J. C. Roberts, N. Kapernaum, F. Giesselmann and R. P. Lemieux, J. Am. Chem. Soc., 2008, 130, 13842 CrossRef CAS PubMed.
- F. Goc, C. Blanc, V. Lorman, M. Nobili, S. Samaritani, G. Galli and W. Kuczynski, Ferroelectrics, 2006, 343, 101 CrossRef CAS.
- K. Saunders, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 80, 11703 CrossRef PubMed.
- S. K. Prasad, D. S. Shankar Rao, S. Sridevi, J. Naciri and B. R. Ratna, J. Phys.: Condens. Matter, 2011, 23, 1 Search PubMed.
- (a) M. V. Gorkunov, M. A. Osipov, J. P. F. Lagerwall and F. Giesselmann, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 76, 51706 CrossRef CAS PubMed; (b) M. V. Gorkunov, F. Giesselmann, J. P. F. Lagerwall, T. J. Sluckin and M. A. Osipov, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 75, 60701 CrossRef CAS PubMed.
- (a) K. Saunders, D. Hernandez, S. Pearson and J. Toner, Phys. Rev. Lett., 2007, 98, 197801 CrossRef PubMed; (b) K. Saunders, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 77, 61708 CrossRef PubMed.
- Y. Shen, L. Wang, R. Shao, T. Gong, C. Zhu, H. Yang, J. E. Maclennan, D. M. Walba and N. A. Clark, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 88, 062504 CrossRef PubMed.
- S. K. Prasad, D. S. Shankar Rao, S. Sridevi, C. V. Lobo, B. R. Ratna, J. Naciri and R. Shashidhar, Phys. Rev. Lett., 2009, 102, 147802 CrossRef PubMed.
- R. Shashidhar, B. R. Ratna, G. G. Nair and S. K. Prasad, Phys. Rev. Lett., 1988, 61(5), 547 CrossRef CAS PubMed.
- L. Li, C. D. Jones, J. Magolan and R. P. Lemieux, J. Mater. Chem., 2007, 17, 2313 RSC.
- J. C. Roberts, N. Kapernaum, F. Giesselman and R. P. Lemieux, J. Am. Chem. Soc., 2008, 130, 13842 CrossRef CAS PubMed.
- J. C. Roberts, N. Kapernaum, Q. Song, D. Nonnenmacher, K. Ayub, F. Giesselmann and R. P. Lemieux, J. Am. Chem. Soc., 2010, 132, 364 CrossRef CAS PubMed.
- Q. Song, D. Nonnenmacher, F. Giesselmann and R. P. Lemieux, J. Mater. Chem. C, 2013, 1, 343 RSC.
- C. P. J. Schubert, C. Muller, M. D. Wand, F. Giesselmann and R. P. Lemieux, Chem. Commun., 2015, 51, 12601 RSC.
- R. J. Birgeneau, C. W. Garland, A. R. Kortan, J. D. Litster, M. Meichle, B. M. Ocko, C. Rosenblatt, L. J. Yu and J. Goodby, Phys. Rev. A: At., Mol., Opt. Phys., 1983, 27, 1251 CrossRef CAS.
- L. D. Landau, Statistical Physics, Pergamon Press, Oxford, 3rd edn, 1980 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10028g |
| ‡ Authors have equal contribution. |
| This journal is © The Royal Society of Chemistry 2016 |



