A review on the effect of bioethanol dilution on the properties and performance of automotive lubricants in gasoline engines
L. S. Khuong
*,
N. W. M. Zulkifli
,
H. H. Masjuki
,
E. Niza Mohamad
,
A. Arslan
,
M. H. Mosarof
and
A. Azham
Department of Mechanical Engineering, Faculty of Engineering, University of Malaya, 50603 Lembah Pantai, Kuala Lumpur, Malaysia. E-mail: sokhuongum@gmail.com; Fax: +60 379675317; Tel: +60 173238647
First published on 6th July 2016
Abstract
Owing to the growing concern over the depletion of fossil fuels and the increasing rate of greenhouse gas emissions which will lead to global warming, many researchers are now dedicated to producing alternative biofuels in order to help address the above-mentioned issues. Bioethanol is one of the biofuels which has gained much attention for use in existing gasoline engines and nowadays, bioethanol is blended with gasoline at higher proportions since the use of bioethanol helps reduce exhaust emissions such as soot, carbon oxides and unburned hydrocarbons. However, the use of bioethanol has undesirable effects on the tribological properties of the fuel blend, and it is possible that the automotive lubricant will be contaminated with diluted oxygenated bioethanol during engine operations. Moreover, the addition of bioethanol into gasoline alters the properties of the fuel, which in turn affects the vehicle performance. Since bioethanol has a significantly higher boiling point and latent heat of vaporization compared to gasoline, it is likely that the level of bioethanol dilution in the automotive lubricant will increase significantly, which in turn degrades the quality of the lubricant to protect the engine components against friction and wear. The purpose of this review paper is to highlight the physicochemical properties of bioethanol and its blends as well as the effect of bioethanol dilution on the properties and performance of automotive lubricants in gasoline engines. Based on the key findings, it can be concluded that bioethanol dilution has a significant effect on the properties of automotive lubricants, particularly on oil consumption, corrosion, wear and sludge, which will lead to engine failure. However, the contamination of automotive lubricants can be prevented by the addition of additives such as dispersants, detergents or antioxidants, which will improve the lubricity and performance of the engine oil.
1. Introduction
Nowadays, gasoline and diesel engines are widely used throughout the globe for transportation, electricity generation, construction and industry as well as agriculture. It is projected that the number of vehicles will increase from 1.3 billion to 2.0 billion between 2030 and 2050.1 However, the growth of accessible base oil and gas production does not match the projected demand rate from 2040 to 2050 since it is forecasted that the demand rate will increase to 105 Mb per day in 2030 and the demand rate will increase further in the following years.2 The ever-increasing number of vehicles which make use of fossil fuels over the years has led to an increase in the fuel price and environmental issues as well as the depletion of non-renewable fossil fuels. In order to address these current issues, much effort is being made to develop alternative fuels from renewable and sustainable sources as well as exploring their properties. Bioethanol and its blends are among the biofuels that have gained much attention due to the availability of agricultural residues which can be used for bioethanol production.Bioethanol has been widely used around the world, and it is typically blended with other fuels such as gasoline, diesel and biodiesel.3 USA is now the leading producer of bioethanol in the world, whereby 18.3 billion litres of bioethanol was produced by USA in 2006.4 Most vehicles now use 10–15% of ethanol with gasoline, and the so-called ‘biofuel’ engines are typically designed to run on E85 blends which are made up of 85% ethanol and 15% gasoline.5 Interestingly, in Brazil, flex-fuel engines are designed such that they can be used with any bioethanol–gasoline blends, regardless of the proportion of bioethanol.6 This helps reduce carbon emissions and particulate mass concentrations in the vehicle exhaust.7,8 According to the National Association of Automotive Vehicle Manufacturers, more than 85% of flex-fuel vehicles are manufactured in Brazil.9 Both Sweden and Belgium aim to increase the use of bioethanol as transportation fuel.10,11 More importantly, ethanol has become one of the alternative fuels of interest in Sweden ever since the 1990s. The annual use of ethanol was 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 in 2001. About 3% of the public service bus fleet (more than 400 buses) use ethanol as fuel, and about 4450 flex-fuel vehicles run on E85 blends.12 Cambodia, one of the developing countries in Southeast Asia, aims to reduce imported fossil fuels. 10 and 20% of fossil fuels such as diesel and gasoline will be replaced with biodiesel and bioethanol, respectively, by year 2030.13
000 m3 in 2001. About 3% of the public service bus fleet (more than 400 buses) use ethanol as fuel, and about 4450 flex-fuel vehicles run on E85 blends.12 Cambodia, one of the developing countries in Southeast Asia, aims to reduce imported fossil fuels. 10 and 20% of fossil fuels such as diesel and gasoline will be replaced with biodiesel and bioethanol, respectively, by year 2030.13
Bioethanol offers a variety of benefits. Firstly, bioethanol can be derived from various feedstocks such as sugar cane, corn and wheat (i.e. first-generation bioethanol), lignocellulosic materials (i.e. second-generation bioethanol) and algal biomass (i.e. third-generation bioethanol).14 Secondly, bioethanol burns with a smokeless blue flame and it has a low auto-ignition temperature. The auto-ignition temperature is the temperature at which the bioethanol will ignite spontaneously in ambient conditions without external source of ignition such as flame or spark.15 Thirdly, bioethanol is miscible with water and most organic solvents such as acetic acid, acetone, ether, ethylene glycol, glycerol and toluene.16,17 Lastly, the addition of bioethanol into gasoline or diesel can help reduce the concentration of particulate mass in the exhaust and this helps reduce carbon oxide emissions due to the high octane number and oxygen content of bioethanol–gasoline or bioethanol–diesel blends. Bioethanol addition also helps increase the air–fuel ratio in the fuel-rich region, which results in complete fuel combustion and reduction of carbon oxide emissions.18
Even though bioethanol is a good alternative fuel, it is corrosive to engine components due to its high oxygen content.19 The presence of water in the chemical structure of alcohol has a corrosive effect on fuel systems and results in phase separation, and this is undesirable for engine operations.20 Corrosion causes damage to the components in the fuel system since these components are made of metals such as steel and zinc–aluminium alloys. Corrosion also causes damage to fuel pumps and fuel tanks.21 More importantly, it has been shown that bioethanol accelerates corrosion on iron compared to gasoline.22 One study was carried out to investigate the corrosion of metals by using weight loss (immersion test) and electrochemical impedance stereoscopy (EIS), and the results showed that the addition of ethanol into gasoline corrodes the metal components in the fuel system. The results also showed that the level of corrosion increases with an increase in the percentage of ethanol and water in gasoline.23 Baena et al.24 studied the corrosion of various metallic materials used in the automotive industry resulting from the use of bioethanol. They prepared fuel samples by blending 20% bioethanol with gasoline and conducted immersion tests. The results revealed that copper and carbon steel have higher tendency towards corrosion. Hence, they recommended that this fuel blend should not be in direct contact with engine components.
Bioethanol dilution in automotive lubricants also becomes an issue whenever bioethanol is used as the fuel. Automotive lubricants play a significant role to lubricate metal-to-metal contacts and moving parts inside the engines. However, when bioethanol is used as the fuel, there is a possibility that the engine oil will be contaminated with bioethanol. One of the causes of bioethanol dilution is partial combustion of the fuel, whereby the reactive compounds produced from the combustion process dilute into the crankcase through the cylinder liner and mix with the lubricant. This is undesirable since bioethanol dilution significantly alters the properties of the automotive lubricant.25 The immiscible alcohol forms a thick emulsion with the lubricant, which will likely increase wear in engines fuelled by bioethanol.26
The accumulation of bioethanol in the crankcase degrades the quality of engine oil and affects the ability of the engine oil to protect the engine components against friction, wear and formation of deposits resulting from oxidation and corrosion.27 Issues pertaining to the degradation of lubricants have also led researchers to probe deep into the tribological effect of various fuels on the properties and performance of automotive lubricants. Ljubas et al.28 investigated the effect of gasoline dilution on the viscosity of lubricating oils. They studied the dilution of multi-grade synthetic oil (SAE 5W-30) and mineral engine oil (SAE 15W-40) with gasoline (ES 95). The dilution was within a range of 0–10 wt% of fuel in the fuel-oil mixture. The results showed that the amount of ES 95 has an effect on the gradation of mineral engine oils. 3 wt% of ES 95 is undesirable for SAE 40 (15W-40) to retain its SAE gradation. This is due to the fact that the viscosity of the mineral engine oil decreases from 15.02 to below 12.5 mm2 s−1. In contrast, the critical dose of ES 95 is 5 wt% for synthetic oil, resulting in oil inadequacy for the proposed SAE classification. The viscosity of the synthetic engine oil decreases from 11.76 to 9.3 mm2 s−1.
Dhar et al.29 studied the effect of 20% Karanja biodiesel blends on the properties of the lubricating oil in a compression ignition engine operated in 200-hour endurance test. The results showed that there is an increase in the density, carbon residues and ash content of the lubricating oil when the engine is fuelled by the biodiesel blend compared to neat diesel. The higher amount of resinous polymerized materials in the lubricating oil indicates that it is very likely that the lubricating oil undergoes higher oxidation and polymerization. The density and viscosity of the lubricating oil began to decrease after 20 hours and 100 hours due to fuel dilution, resulting in wear of metals such as iron, aluminium, copper, chromium and magnesium. This clearly indicates the deterioration in the quality and ability of the lubricating oil. George et al.30 investigated the effect of diesel soot on the viscosity of the lubricant within a temperature range of 40–90 °C. They observed the interaction between diesel soot and a lubricant additive, i.e. zinc dialkyl dithiophosphate (ZDDP). The results revealed that the viscosity of the oil sample increases with an increase in diesel soot within the temperature range investigated in this study. The increase in oil viscosity in turn causes oil pumpability problems such as clogged fuel pumps and injectors which further result in insufficient lubrication and an increase in engine wear due to metal-to-metal contacts.
It can be deduced that bioethanol has been used as an alternative fuel throughout the world to replace fossil fuels and to reduce the concern over environmental issues, yet bioethanol has not completely become a promising biofuel because its ability to contaminate automotive lubricants is still a significant problem. A large number of experimental studies have been carried out over the years to investigate the effect of fuels on the properties of automotive lubricants. Since the amount of bioethanol has a significant effect on the quality of the fuel blend (particularly on tribological properties), it is deemed important to provide a review on the physicochemical properties of bioethanol and its blends and more importantly, the effects of bioethanol dilution on the properties and performance of automotive lubricants. It is believed that this review paper will provide useful insights about the current works pertaining to bioethanol and bioethanol dilution and is therefore beneficial to researchers and practitioners in this field.
2. Bioethanol as an alternative fuel
2.1. Physicochemical properties of bioethanol
Bioethanol is ethyl alcohol or grain alcohol biofuel having the following chemical formula, CH3–CH2–OH.31 Bioethanol has shown great potentials for use as an alternative fuel in spark-ignition and compression-ignition engines due to its favourable physicochemical properties. The physicochemical properties of bioethanol are summarized in Table 1. Bioethanol is an oxygenated fuel and therefore, it can reduce particulate emissions from engines. Bioethanol also has higher octane number, higher heat of vaporization and broader flammability limits, which enhance fuel combustion, increase compression ratio and shorten ignition timing compared to gasoline and diesel. In brief, bioethanol is more efficient compared to gasoline and diesel.32| Fuel property | Unit | Bioethanol [C2H5OH] |
|---|---|---|
| Density at 15 °C | kg m−3 | 790 |
| Kinematic viscosity at 40 °C | mm2 s−1 | 1.13 |
| Oxygen | Mass% | 34.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Cetane number | — | 5, 8 |
| Octane number | — | 110 |
| Latent heat of vaporization | MJ kg−1 | 0.91 |
| Lower calorific value | MJ kg−1 | 25.22, 26.70 |
| Flash point | °C | 13 |
| Auto-ignition temperature | °C | 332.8, 366.0 |
| Water content | mg kg−1 | 2024 |
| Stoichiometric fuel/air ratio | — | 1/9.01 |
| References | 1 and 34–39 | |
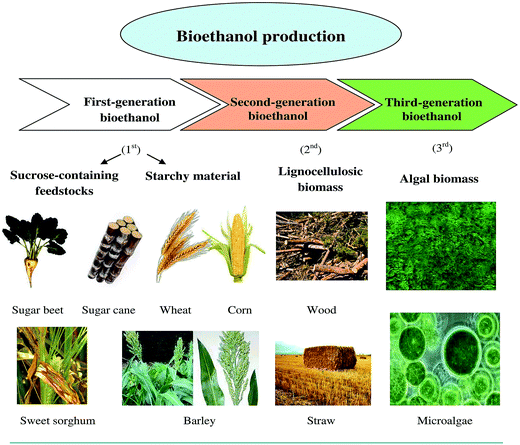
Bioethanol can be produced from renewable feedstocks such as corn, sugar cane, cassava, waste biomass, molasses, starch and cellulose. The feedstocks used for bioethanol production are shown in Fig. 1. In general, there are three types of bioethanol based on the feedstock used for bioethanol production, namely first-generation bioethanol, second-generation bioethanol and third-generation bioethanol.33 First-generation bioethanol is produced from edible feedstocks such as seeds, grains, corn, wheat and sugar cane, which have high sugar or starch content. The sugar or starch is converted into bioethanol through fermentation process. However, it is also known that the production of bioethanol from edible feedstocks is undesirable since food sources are used for fuel production and this leads to food scarcity, especially in developing countries. For this reason, second-generation bioethanol is produced from non-edible feedstocks such as lignocellulosic biomass and crop waste residues including rice husk, straws, grasses and wood. These materials are presently in demand for biofuel production owing to the rising need for energy production from renewable sources. Third-generation bioethanol, however, is produced from algae which are microorganisms that convert sunlight, water and carbon dioxide into energy via photosynthesis. Algae require these components for high algal growth. Algae are desirable for bioethanol production because they require low input and they give high yields of up to 30–100 times more as well as their high oil/lipid, carbohydrate and protein content.33
2.2. Advantages and disadvantages of bioethanol
Bioethanol offers a number of advantages, which is why it is an attractive alternative fuel for use in gasoline and diesel engines. The advantages of bioethanol are listed as follows:• Bioethanol has higher octane number and oxygen content which are desirable for gasoline and diesel engines. In addition, the burning of bioethanol results in lower soot, carbon oxide, unburned hydrocarbon emissions compared to fossil fuels.40
• Bioethanol burns cleanly due to the higher octane content, producing cleaner emissions. This reduces the amount of octane additives.41
• The use of bioethanol–gasoline blends such as E85 can help reduce greenhouse gas emissions.20
• Bioethanol has broader flammability limits, higher flame speed, higher heat of vaporization, higher compression ratio and shorter ignition timing, which enhance fuel combustion compared to fossil fuels.
• The combustion of bioethanol produces emissions which are less reactive to sunlight compared with gasoline. Hence, the use of bioethanol is less detrimental to the environment and ozone layer compared to fossil fuels.41
• Bioethanol is biodegradable and it does not contain aromatic compounds such as olefins and diolefins. Furthermore, bioethanol is less toxic compared to fossil fuels.42
• The use of bioethanol helps provide energy security since it reduces oil imports from foreign countries owing to the fact that bioethanol can be produced domestically. Countries that do not have access to crude oil can grow crops for energy use and attain economic freedom.
However, bioethanol also has several disadvantages which are listed as follows:
• Bioethanol is miscible with water and therefore it has a corrosive effect on engine components.
• Bioethanol has an undesirable effect on electric fuel pumps since it increases internal wear and generates spark.
• Bioethanol has lower heating value and lower energy density, which leads to higher fuel consumption in order to generate the same engine power output as conventional fossil fuels.
• Pure ethanol is difficult to vaporize, which leads to difficult vehicle start-ups in cold weather.
• Bioethanol has higher tendency to reach crankcase since it has higher heat of vaporization compared to fossil fuels and therefore contaminates engine oil.
2.3. Global bioethanol production
Bioethanol has gained much attention from governments all over the world and this is spurred by the availability of feedstocks and facilities for bioethanol production. Much effort is being made to improve the quality and quantity of bioethanol for use in engines.31 Kim and Dale43 reported that the total production of bioethanol from lignocellulose materials is approximately 442 billion litres, which is roughly 16 times as great as the global production of bioethanol in 2004. The world's leading producers of bioethanol are USA and Brazil, followed by Germany, France, Spain, Italy and UK. Bioethanol is increasingly used in USA and Brazil over the years. The bioethanol produced by the USA and Brazil constitutes 86% of the world's bioethanol in 2010. Bioethanol production has been increasing steadily in Europe ever since the biofuel directive was enforced in 2003. European countries produced approximately 576![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 of bioethanol in 2004 and bioethanol production reached 4.6 million m3 in 2013, whereby 19% was produced in Germany. Bioethanol consumption is expected to rise annually in Europe with the implementation of the ‘Renewable Energy Directive’ which prescribes a mandatory bioethanol blending rate of 10% in the transportation sector by 2020.44 There is also a great potential for bioethanol production in China, India, Japan and Indonesia due to the availability of various feedstocks. Asia is now the largest potential producer of bioethanol from crop wastes and residues. The favourable feedstocks for bioethanol production in Asia are rice straws, rice husk, wheat straws and corn stover.43 It has been recorded that the world's bioethanol production for fuel application reached 22
000 m3 of bioethanol in 2004 and bioethanol production reached 4.6 million m3 in 2013, whereby 19% was produced in Germany. Bioethanol consumption is expected to rise annually in Europe with the implementation of the ‘Renewable Energy Directive’ which prescribes a mandatory bioethanol blending rate of 10% in the transportation sector by 2020.44 There is also a great potential for bioethanol production in China, India, Japan and Indonesia due to the availability of various feedstocks. Asia is now the largest potential producer of bioethanol from crop wastes and residues. The favourable feedstocks for bioethanol production in Asia are rice straws, rice husk, wheat straws and corn stover.43 It has been recorded that the world's bioethanol production for fuel application reached 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 966.87 million gallons in 2011.45 Fig. 2 shows the global production of bioethanol from 2007 to 2015. It is evident that there is a gradual increase in the global bioethanol production within this period, whereby the total production of bioethanol is more than 25 billions of gallons in 2015. It is projected that the total bioethanol production may reach or exceed 125 billion litres by 2020 owing to the new targets set by the governments in America, Brazil, Asia and Europe.46
966.87 million gallons in 2011.45 Fig. 2 shows the global production of bioethanol from 2007 to 2015. It is evident that there is a gradual increase in the global bioethanol production within this period, whereby the total production of bioethanol is more than 25 billions of gallons in 2015. It is projected that the total bioethanol production may reach or exceed 125 billion litres by 2020 owing to the new targets set by the governments in America, Brazil, Asia and Europe.46
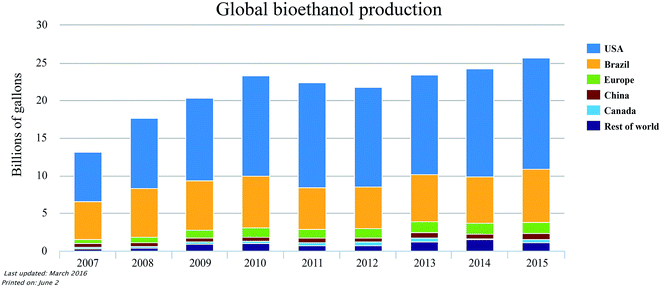 | ||
| Fig. 2 Global bioethanol production from 2007 to 2015. Retrieved from Renewable Fuel Association.47 | ||
2.4. Bioethanol blends
Bioethanol can be blended with gasoline, diesel and biodiesel, and these blends can be used to fuel gasoline and diesel engines without any engine modifications or with minor engine modifications.48 The physicochemical properties of bioethanol, diesel, gasoline and biodiesel are summarized in Table 2. In general, it is recommended that bioethanol is blended with gasoline at a concentration of 10 vol%. This blend is designated as E10.26 Bioethanol–gasoline blends are often used in fuel injection engines of light-duty vehicles as alternative fuels to gasoline or as fuel additives due to their higher octane number, faster flame speed, higher heat of vaporization and broader flammability limits. These properties allow the engine to achieve higher compression ratio and shorter combustion time, which help reduce engine emissions compared to pure gasoline.31 E10 contains around 3.5 wt% of oxygen and this blend is typically prepared by ‘splash’ blending ethanol with gasoline that is already in the fuel distribution system. It shall be noted here that bioethanol can be blended with unleaded gasoline to fulfil the octane requirements of high-grade gasoline. Moreover, bioethanol can be blended with reformulated gasoline (RFG) up to an oxygen level of 2 wt%. However, the formula of the final fuel must fulfil all fuel specifications including the vapour pressure for RFG.32 Bioethanol is also typically blended with diesel though this is mostly done in laboratory experiments. This is due to the fact that bioethanol is immiscible with diesel over a wide range of temperatures and water content, which results in phase separation between bioethanol and diesel/biodiesel during engine operations. This is why bioethanol–diesel blends are not highly recommended for use in diesel engines. However, phase separation can be prevented by using an emulsifier which will suspend small droplets of ethanol within the diesel fuel or by using a co-solvent which will maintain solubility32 and ensure molecular compatibility and bonding. This results in a homogeneous blend.49 Bioethanol–gasoline and bioethanol–diesel blends are common binary blends as engine fuels. Bioethanol can also be blended with diesel and biodiesel (diesel–biodiesel–bioethanol). Such blends are known as tertiary blends. D80B10E10 is an example of a tertiary blend, which consists of 10% biodiesel, 10% bioethanol and 80% diesel.| Fuel property | Unit | Bioethanol | Diesel | Gasoline | Biodiesel |
|---|---|---|---|---|---|
| Density at 15 °C | kg m−3 | 790.0 | 837.3 | 737.0 | 880.0 |
| Kinematic viscosity at 40 °C | mm2 s−1 | 1.130 | 2.780, 3.180 | 0.593 | 6.000 |
| Cetane number | — | 5, 8 | 47.64, 53.90 | 10–15 | 47–65 |
| Oxygen | Mass% | 34.7 | — | 0–4 | 11.0 |
| Octane number | — | 110 | 10–30 | 86–94 | −25 |
| Latent heat of vaporization | kJ g−1 | 921.1 | 370.0 | 289.0 | 330.0 |
| Calorific value | MJ kg−1 | 25.22, 26.70 | 43.80 | 34.84 | 39.40 |
| Flash point | °C | 13 | 64, 74 | −43 | 170 |
| Auto-ignition temperature | °C | 332.8, 366.0, 420.0 | 230.0 | 257.0 | 225.0 |
| Water content | mg kg−1 | 2024 | 50 | — | — |
| Stoichiometric fuel/air ratio | — | 1/9.01 | 1/14.67 | 1/14.7 | 1/13.8 |
| References | 1, 35, 37–39 and 50–52 | 35, 39, 48, 53 and 54 | 45, 51, 52 and 55–57 | 52, 54 and 58 |
2.5. Physicochemical properties of bioethanol blends
Even though bioethanol blends are generally favourable alternative fuels, there is still a critical need to investigate and enhance the physicochemical properties of bioethanol blends. The addition of bioethanol into gasoline and diesel alters the properties of the fuel blend, which in turn affects engine operations. In general, the presence of bioethanol reduces the physicochemical properties of the fuel blend in diesel engines.22 The physicochemical properties of several biodiesel–diesel blends, bioethanol–gasoline blends and bioethanol–diesel–biodiesel blends are summarized in Tables 3–5, respectively. It can be seen that blending various fuels alters the kinematic viscosity, density, cetane number, octane number, heating value, heat of vaporization, volatility and other properties of the fuel blend.22,38,59 This in turn affects the lubricity, engine performance, exhaust emissions of the fuel blend and lubricant performance due to fuel dilution into oil sump.60| Fuel property | Unit | Bioethanol–diesel blends | ||||
|---|---|---|---|---|---|---|
| E5D95 | E10D90 | E15D85 | E20D80 | E25D75 | ||
| Density at 15 °C | kg m−3 | 834.3 | 831.7 | 829.4 | 815.0 | — |
| Kinematic viscosity at 40 °C | mm2 s−1 | 2.53 | 2.31 | 2.19 | 3.00 | — |
| Cetane number | — | — | 44.6 | 44.1 | 36.0 | — |
| Octane number | — | — | — | — | — | — |
| Calorific value | MJ kg−1 | 43.6318 | 43.1925 | 42.7448 | 41.8745 | 41.0042 |
| Flash point | °C | 24 | 25 | 27 | 25 | 25 |
| Water content | mg kg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
100 | 1300 | 140 | — | — |
| References | 37, 48 and 53 | 37, 48 and 53 | 37, 48 and 53 | 48, 53 and 55 | 48 and 53 | |
| Fuel property | Unit | Bioethanol–gasoline blends | |||||
|---|---|---|---|---|---|---|---|
| E0 | E10 | E15 | E20 | E25 | E30 | ||
| a Denotes typical or calculated value. | |||||||
| Density at 15.6 °C | kg m−3 | 740.0 | 739.6 | 749.5 | 754.1 | 757.1 | 761.3 |
| Kinematic viscosity at 30 °C | mm2 s−1 | 0.4872 | 0.5383 | 0.5619 | 0.6007 | 0.6380 | 0.6614![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Oxygen content | Mass% | — | — | — | — | — | — |
| Octane number | — | 93.2 | 97.1 | 98.6 | 100.4 | 99.5 | 102.5 |
| Heat of combustion | MJ kg−1 | 34.84 | 33.19 | 32.91 | 32.43 | 31.70 | 31.53 |
| Flash point | °C | — | 29.0 | 29.4 | 29.5 | 30.0 | 29.2 |
| Fire point | °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
25.0 | 29.0 | 29.1 | 30.0 | 32.0 | 30.2 |
| Stoichiometric air/fuel ratio | (Weight)a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
14.70 | 14.30 | — | 13.56 | — | — |
Heat of vaporization![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
kJ g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
— | — | — | — | — | — |
References![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
36, 45 and 56 | ||||||
| Sample no. | %D | %B | %E | Cetane index | Flash point (°C) | Pour point (°C) | Density (g ml−1) | Heat of combustion (MJ kg−1) |
|---|---|---|---|---|---|---|---|---|
| a Note: D, B and E denotes diesel, biodiesel and bioethanol, respectively. | ||||||||
| 1 | 90 | 10 | 0 | 47.99 | 71.00 | 6 | 0.8388 | 44.7 |
| 2 | 90 | 5 | 5 | 47.31 | 17.50 | 3 | 0.8318 | 44.5 |
| 3 | 90 | 0 | 10 | 46.05 | 14.50 | 3 | 0.8268 | 43.4 |
| 4 | 85 | 15 | 0 | 48.52 | 73.50 | 6 | 0.8417 | 44.2 |
| 5 | 85 | 10 | 5 | 47.70 | 14.00 | 3 | 0.8334 | 43.7 |
| 6 | 85 | 5 | 10 | 46.67 | 13.50 | 3 | 0.8313 | 43.6 |
| 7 | 85 | 0 | 15 | 45.81 | 13.00 | 3 | 0.8247 | 42.5 |
| 8 | 80 | 15 | 5 | 48.66 | 16.00 | 3 | 0.8375 | 43.3 |
| 9 | 80 | 10 | 10 | 46.85 | 15.00 | 3 | 0.8331 | 43.5 |
| 10 | 80 | 5 | 15 | 46.25 | 13.00 | 3 | 0.8290 | 42.8 |
| 11 | 100 | 0 | 0 | 47.64 | 69.00 | 6 | 0.8354 | 45.0 |
| 12 | 0 | 100 | 0 | 55.40 | 122.00 | 9 | 0.8786 | 39.6 |
| 13 | 0 | 0 | 100 | 5–8 | 13.00 | −117.30 | 0.7940 | 27.0 |
| Property | Importance |
|---|---|
| Kinematic viscosity | Injector wear, spray pattern (poor fuel dispersion), pump wear, pump resistance, filter damage, formation of engine deposits |
| Cetane number | Engine knock and abnormal combustion (combustion roughness) |
| Volatility | Poor cold start performance, deposits, exhaust smoke |
| Sulfur content | Emissions control devices, engine wear and deposit |
| Water content | Filter plugging, injector wear, increased corrosion |
| Lubricity | Injector and pump wear |
| Ash content | Injector and fuel pump wear, piston-ring wear, engine deposits |
| Corrosion | Deterioration in engine components |
| Carbon residue | Formation of deposits in fuel system and combustion chamber |
| Property | Units | Limits | Test method |
|---|---|---|---|
| Kinematic viscosity, 40 °C | mm2 s−1 | — | ASTM D445 |
| Density, 15 °C | kg m−3 | 715–770 | ASTM D4052 |
| Reid vapor pressure (RVP), 37.8 °C | kPa | 54–103 | ASTM D323 |
| Research octane number (RON) | — | Min. 95.0 | ASTM D2699 |
| Distillation temperature: T10 | °C | Max. 70 | ASTM D86 |
| T50 | °C | 66–110 | ASTM D86 |
| T90 | °C | Max. 190 | ASTM D86 |
| Oxidation stability, 110 °C | Minutes | Min. 240 | ASTM D525 |
| Oxygen content | Volume% | 5.5–11.0 | ASTM D4815 |
| Lead content | g L−1 | Max. 0.013 | D3237 or D5059 |
| Benzene | Volume% | Max. 3.5 | ASTM D4420 |
| Sulfur content | Mass% | Max. 0.0080 | ASTM D 381 |
| Reference | 63 and 64 | ||
| Property | Units | Limits | Test method |
|---|---|---|---|
| Kinematic viscosity, 40 °C | mm2 s−1 | 1.9–4.1 | ASTM D445 |
| Density, 15 °C | kg m−3 | 820–845 | EN ISO 3675 |
| Cetane number, min | — | 40 | ASTM D613 |
| Flash point, min | °C | 52 | ASTM D93 |
| Distillation temperature, 90% volume recovered | °C | 282–338 | ASTM D86 |
| Oxidation stability, max | g m−3 | 25 | EN ISO 12205 |
| Lubricity, 60 °C, WSD, max | μm | 520 | ASTM D6079 |
| Ash, max | Mass% | 0.01 | ASTM D482 |
| Water and sediment, max | Volume% | 0.05 | ASTM D2709 |
| Sulfur, max | Mass% | 0.50 | ASTM D129 |
| Reference | 62, 65 and 66 | ||
Fig. 3 shows the effect of ethanol on the Reid vapour pressure (RVP) of the fuel compared to other oxygenated fuels such as methanol, gasoline-grade tertiary-butyl alcohol (GTBA), methyl tertiary-butyl ether (MTBE) and ethyl tertiary-butyl ether (ETBE). Ethanol will be limited only to base gasoline when there is a strict control on the vapour pressure.32 The cetane number of bioethanol blends is generally dependent on the concentration of ethanol as well as the amount and type of additive used in the fuel blend. The cetane number of the bioethanol blends decreases significantly because bioethanol has a lower cetane number. De-gang Li et al.22 studied about the experimental work done by Corkwell and discovered that blending 10% of ethanol with diesel reduces the cetane number of the fuel blend by 7.1 units. Unlike fossil fuels, bioethanol has the lowest heating value and therefore, when ethanol is added into gasoline or diesel, the heating value of the fuel blend decreases with an increase in the proportion of ethanol. This increases the consumption of bioethanol blends in gasoline and diesel engines.57,67 It is also crucial to determine the water content in bioethanol blends since a high water content results in corrosion, phase separation and microbial growth.22,37 In general, bioethanol can be blended with gasoline at all proportions in most conditions while bioethanol cannot be blended easily with diesel due to its immiscibility.22
 | ||
| Fig. 3 Effect of ethanol on the Reid vapour pressure (RVP) of fuel compared to other oxygenated fuels such as methanol, gasoline grade tertiary-butyl alcohol (GTBA), methyl tertiary-butyl ether (MTBE) and ethyl tertiary-butyl ether (ETBE). Reproduced with permission from ref. 32, copyright 1996, Taylor & Francis. | ||
2.6. Critical summary of bioethanol
It can be described that bioethanol is commonly used at the increasing rates as a substitute for fossil fuels in gasoline engines due to its production availability and significant performance and properties. Mostly, bioethanol is blended into gasoline at the concentration range of 5–30% for SI engine and up to 85% for flex-fuel engines. Bioethanol and its blends reduce the emission and enrich engine performance with a little higher fuel consumption compared to fossil fuels. Bioethanol blends, however, exhibits some major problems concerning material compatibility and immiscibility with other fuels such diesel and biodiesel blends. The high amount of water and organic acid in bioethanol enhance corrosion on the contact of sliding surfaces especially fuel injector and injection pump. Another main problem of bioethanol is its possibility to contaminate automotive lubricant through fuel dilution which is discussed in Section 4. This contamination might be due to some bioethanol properties interacting with lubricant and lubricant additives which actively perform to protect engine components of tribology systems. The diluted fuel in particular bioethanol provokes further oxidation and causes serious problems such as wear, sludge and deposit formation for lubricant and engine. There have not been many researchers investigating thoroughly into the corrosive effect of bioethanol as well as its fuel dilution on lubricant properties and performance yet.3. Automotive lubricants
3.1. Overview of automotive lubricants
At present, there are various types of automotive lubricants commercially available in the market such as mineral oils, semi-synthetic oils, fully synthetic oils and bio-base oil. Each base oil lubricant has different demand and growing trend in the market according to different applications. The future trend of different base oils is shown in Table 9. The mineral oil lubricant has the largest demand in the market because it has low cost and is suitable for use in most applications. Synthetic oil has a high demand in the market due to its good performance in severe and heavy-duty applications. The use of bio-based lubricant has a limited demand, but it has the fastest growing demand in the market compared to other types of lubricant. Nowadays, most modern lubricants may contain chemical additives which enhance the quality and properties of the lubricants and extend service life and performance. Table 10 shows different types of additive and their roles in lubrication. The market demand for global lubricant additives was 4.28 million tons in 2015, and its demand is projected to reach 5.36 million tons in 2024, at a CAGR of 2.5% from 2015 to 2024.69| Base oils | Status and market trend |
|---|---|
| a CAGR = compound annual growth rate. | |
| Mineral oil lubricant | • The mineral oil lubricant accounts for the largest share among all the products of the global lubricants market, at a CAGR of 2.7% from 2016 to 2022 |
• The demand for mineral oil lubricant for automotive segment was 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740.62 kilo tons in 2014, and its demand will reach 25 740.62 kilo tons in 2014, and its demand will reach 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347.84 kilo tons by 2020, at a CAGR of 1.81% 347.84 kilo tons by 2020, at a CAGR of 1.81% |
|
| • Mineral oil lubricant is easily available and highly used in all the sectors, and its future market trend is also expected to be the most dominant lubricant between 2016 and 2021 due to its low cost as compared to other types of lubricants | |
| Synthetic lubricant | • The demand for synthetic lubricant from the consumer automotive application segment is expected reach 11.86 million tons in 2018, at a CAGR of 2.58% from 2013 to 2018 |
| • The world market for synthetic lubricants base stock is projected to grow at an average annual rate of around 3.5% per year on a volume basis to 2016 | |
| • Growth in synthetic lubricant base stocks is expected to continue at a low rate in Europe and Japan. In contrast, high growth rates are expected in China, other Asian countries and South America | |
| Bio-based lubricant | • The demand for bio-based lubricants was 505.6 kilo tons in 2011 and will be 785.0 kilo tons in 2018, recording a CAGR of 6.6% between 2013 and 2018 |
| • For automotive application, bio-based lubricant employment accounted for 25.7% of worldwide demand in 2011 | |
| Reference | 72–74 |
| Additive | Chemical compound | Role |
|---|---|---|
| a ZDDP = zinc dialkyl dithiophosphate, MoDTC = molybdenum dithiocarbamate. | ||
| Deposit control additives | ||
| Detergent | Succinimides | To reduce insoluble deposits formed on surfaces at high temperatures |
| Dispersant | PIB-succinimide | To limit engine sludge and vanish |
| Anti-oxidant | ZDDP | To prevent oxidation of engine oil |
| Anti-wear and EP additives | ZDDP | To reduce wear from corrosion and limit mechanical wear |
| To prevent scuffing of rubbed surfaces under shock and very high loads | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Properties modifiers | ||
| Friction modifiers | MoDTC | To reduce friction of metal-to-metal contact surface |
| Viscosity index enhancers | Olefin copolymer | To limit variation of oil viscosity at high temperature |
| Anti foaming | Silicone | To reduce the formation of foam in engine oil |
| Pour point depressant | Polyalkylmethacrylates | To reduce pour point of oil containing paraffinic compound |
| Corrosion inhibitor | Alkyl sulphonates | To neutralize acid number caused by oxidation of engine oil |
The physicochemical properties of automotive lubricants that are of interest to researchers and practitioners are viscosity, density, boiling point, flash point, oxidation stability, volatility, total base number and wear rate. In general, lubricants can be classified as solid and liquid lubricants. Solid lubricants are used whenever it is important for the lubricants to stay in place. These lubricants are composed of a solid binder as well as additives such as corrosion inhibitors or solvents. Molybdenum, disulphide, hexagonal boron nitride, boric acid, graphite and lubricious oxides are among the common solid lubricants. Liquid lubricants are essential engine oils in the crankcase, which serve to lubricate and protect the engine. High quality lubricants need to have high fluidity at low temperature and minimum change in viscosity at high temperature, high anti-friction characteristic and maximum resistance to oxidation. These lubricants protect engine components such as bearings, pistons, piston rings, cylinder liners and the valve train against wear by forming a chemical film on the surface of the components. More importantly, lubricants protect engine components from corrosion by neutralizing acid using an alkaline agent and they transport waste products or sludge away from the site where they are generated. Lubricants also help improve fuel economy due to the reduction of friction in the engine.70,71 Owing to the increasing use of bioethanol which significantly causes higher fuel dilution, the development of high performance lubricants has been researched to protect against the contaminations especially fuel dilution.
3.2. Types of automotive lubricants
There are three main types of automotive lubricants, namely mineral oils, synthetic lubricants and bio-based lubricants. All of these lubricants are produced to attain satisfactory performance during engine operations as well as resisting the contamination of fuel dilution causing oil degradation, oxidation and corrosion over a period of time during engine operation. In general, the ideal lubricant is one which will give complete protections to the engine components against friction and wear, and it can be used in various operating conditions without a change in its properties. It is generally understood that even though one lubricant may be suitable for a particular application, it may be undesirable for another. For this reason, automotive lubricants are constantly improved to fulfil the requirements of various applications and to achieve better performance which is not attainable from previous lubricants.(a) Improved energy efficiency: synthetic lubricants improve the energy efficiency of engines since it reduces friction of the engine components due to its ability to lower viscosity.
(b) Wide operating temperature range: synthetic lubricants can be used over a wide range of operating temperatures due to its lower pour point, higher viscosity index, better oxidative stability and lower volatility.
(c) Enhanced design ratings: synthetic lubricants enable machines to be uprated to higher power with longer life expectancy.
(d) Reduced maintenance: synthetic lubricants help prevent oxidation and corrosion. This reduces fuel consumption, prolongs the lifespan of components, eliminates summer/winter oil changes and extends oil drain intervals.
(e) Improved reliability and safer operation: synthetic lubricants enhance component reliability especially in racing automobiles.79
However, bio-based lubricants also have several disadvantages. Firstly, these lubricants have poor oxidation stability, or in other words, these lubricants are prone to oxidation. Thus, the user needs to change the lubricant on a frequent basis. Secondly, bio-based lubricants can only be used within a moderate range of temperatures due to their higher pour point and low thermal stability.80,81 However, the degradation of bio-based lubricants by rapid oxidation can be overcome by the addition of additives. Even though these additives help prevent oxidation, this comes at the expense of increased cost. Hence, bio-based lubricants are more expensive than mineral oils. One way to reduce the cost of bio-based lubricants is by the addition of a catalyst. This was done by Lathi and Mattiasson82 who used Amberlyst 15 catalyst to reduce the pour point of a lubricant. Fig. 4 shows the ring opening reaction of epoxidized vegetable oil with n-butanol. It can be seen that the epoxidation reaction occurs to form bonds with the alcohol. The ring opening reaction with 2-ethylhexanol lowers the pour point of the lubricant while it retains the viscosity and biodegradability of the epoxidized vegetable oil. It shall be noted here that the catalyst remains effective so that it can be reused up to four times, which gives greater economic viability. In general, it can be deduced that bio-based lubricants would be the best alternative among all lubricants provided that the high cost of these lubricants can be reduced.
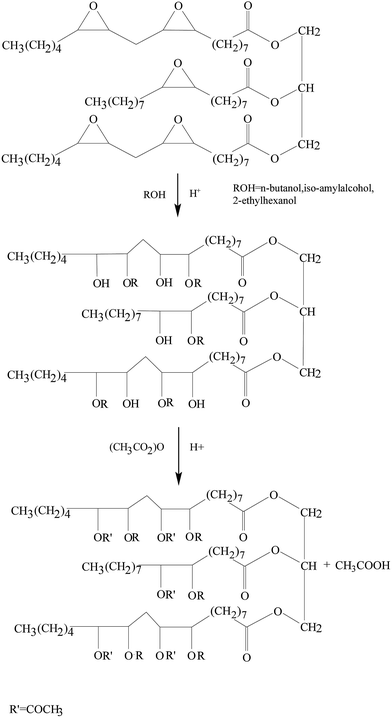 | ||
| Fig. 4 Ring opening reaction of epoxidized vegetable oil with n-butanol. Reproduce with permission from ref. 82, copyright 2006, Elsevier. | ||
3.3. Evolution of lubricants used for gasoline engines
A variety of lubricants have been produced since the 1930s in order to address issues related to friction and wear as well as the formation of deposits and sludge during engine operations. The performance designation of various lubricants for gasoline engines is summarized in Table 11. The designations SA, SB, SC and SD were adopted for spark-ignition engines in 1971. It can be seen from Table 11 that the SA lubricants were used from 1900 to 1930. It shall be noted that these lubricants do not contain any performance additives and they can still be found in some retail establishments. The SB lubricants were used from 1931 to 1963 and they contain a minimum amount of performance additives, whereas the SC lubricants were used from 1964 to 1967. The SC lubricants are likely premium oils which contain a full range of performance additives. In contrast, the SD lubricants contain a higher level of performance additives and these lubricants were mostly used in 1971.71 However, it shall be highlighted that the SA through SH lubricants are now obsolete. This is due to the fact that the engine test which is compulsory to determine whether the lubricants fulfil the standard requirements of oil performance cannot be carried out since the reference oils, fuels and engine components are no longer available.83The SE, SF, SG, SH, SJ and SL lubricants were developed from 1972 to 2001 and these lubricants were formulated to provide greater engine protections. The SL lubricants were developed to prevent oil thickening at high temperatures, whereas the SF lubricants were developed to protect the engine components against wear. The SG lubricants were developed to prevent oil oxidation and the formation of sludge in the engines.71 The SL/ILSAC GF-5, SM and SN lubricants were introduced in 2010 for vehicles manufactured in 2011 as well as older vehicles. Both the GF-5 and SN lubricants which are still in use are formulated to provide high-temperature deposit protection for pistons and turbochargers as well as improving sludge control. These lubricants help improve fuel economy and control engine emissions, and they are compatible with engines operating on bioethanol blends up to E85.
All types of lubricants can be suffered not only from the external contaminants (dirt, water, air, etc.) but also from fuel contaminants such as the entry of gasoline, diesel, bioethanol and biodiesel through cylinder liner. Mineral-based oils are commonly used in vehicle engines due to their low cost, but they cannot resist fuel dilution which can make mineral-base oil degrade and lose their performance and quality easily. Various additives such as corrosion inhibitor, anti-wear and anti-oxidant are used in mineral-based oil to enhance the performance in term of corrosion and oxidation. Synthetic oils have higher viscosity index and oxidation stability compared to mineral oils, which enable synthetic oils to combat serious fuel contamination. The internal chemical compounds of fuels in particular biofuels may interact with chemical compounds of lubricants and lubricant additives and provoke further oxidation or chemical reactions. Compared to other fuels, bioethanol contains higher oxygen content and water content with slightly lower acid value. These properties enable lubricants to degrade quickly and form organic acid which aggressively attacks engine components. It also forms deposit and sludge when it polymerizes and oxidizes in the engine oils.
4. Fuel dilution in automotive lubricants
Fig. 5 shows that fuels affect not only the engine but also the lubricant in piston ring-cylinder system. Fuel dilution is a serious issue for automotive lubricants, particularly for direct-ignition engines. Fuel dilution is the condition in which unburned fuel accumulates in the engine crankcase through the cylinder walls. In reality, the fuel does not burn completely inside the combustion chamber of gasoline and diesel engines for various reasons. A high proportion of unburned fuel leaves the engine as the exhaust while a small proportion of the unburned fuel impinges on the cold walls of the combustion chamber, which will then be scrapped to the oil pan and mix with the lubricant.84 It has been identified that the causes of fuel dilution are the mode of injection and the properties of the fuel.85,86 It has been shown in previous studies that even a low level of fuel dilution can degrade the physicochemical properties of the lubricant such as viscosity, total base number (TBN), total acid number (TAN), flash point and oxidation stability as well as the concentration of the engine oil additives. This in turn affects the lubricating properties of the lubricant.84,87 | ||
| Fig. 5 The effect of biofuel and lubricant on the engine. Credits taken from A. G. Sappok (MIT). Reproduced with permission from ref. 88, copyright 2011, John Wiley & Sons. | ||
4.1. Primary causes of fuel dilution in automotive lubricants
A low level of fuel dilution in automotive lubricants is common in normal engine operations. Fuel dilution is acceptable, provided that it is within tolerance limits since the level of fuel dilution will have a significant effect on the physicochemical properties of the lubricant. It has been mentioned previously that the primary causes of fuel dilution in lubricants are the mode of injection and properties of the fuel.One of the serious side-effects of the pre-injection and post-injection strategy in direct-injection gasoline and diesel engines is the increase in fuel dilution of the lubricant.89 The post-injection of fuel used for regeneration of the diesel particulate filter accelerates fuel dilution. Filter regeneration increases fuel dilution in the lubricant because it involves periodic injection of a small quantity of fuel with the burned fuel in order to increase the exhaust temperature and burn off all unburned hydrocarbons at the exhaust.90 Zdrodowski et al.87 discovered that post-injection results in 20 wt% fuel dilution before the oil was drained. Therefore, the engine oil was not suitable for use in the engine. A direct injection engine has a fuel injector in the combustion chamber, whereby the fuel is injected directly towards the upper side of the piston and cylinder wall. Consequently, the fuel mixes with the oil film on the cylinder wall. In general, a greater fuel dilution occurs at low temperatures in multi-point injection (MPI) engines, which reduces the viscosity of the lubricant. Late injection also affects the level of fuel dilution.91 The main disadvantage of late injection is that it increases the amount of lubricant exposed to the fuel. Unlike normal combustion, late-injected fuels do not atomize and evaporate easily. Hence, a portion of this fuel dilutes through the cylinder wall and washes down with the lubricant into the sump. This degrades the quality of the lubricant, which leads to engine wear and/or engine failure.86 If the fuel is injected late during the power stroke, there is a higher chance that the fuel spray will hit the oil on the cylinder liner rather than the piston bowl, resulting in an increase in fuel dilution.85 Fig. 6 shows fuel dilution of the lubricant based on the mode of injection. One study proved that the turbocharged gasoline direct injection (TGDI) engine operated at high-speed end torque results in a significant fuel dilution. This is due to the increased injection timing, which results in large fuel drops impinging on the top of the piston. The results also showed that the higher the torque rate-power, the greater the level of fuel dilution.92
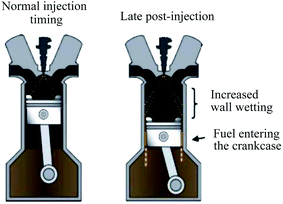 | ||
| Fig. 6 Fuel dilution of lubricants based on the mode of injection. Reproduced with permission form ref. 86, copyright 2013, SAE International. | ||
The fuel properties are also the cause of fuel dilution. It is worth noting that the effect of bioethanol on the fuel dilution differs from that of gasoline. Based on this literature review, it is found that most of the studies related to the effect of fuel properties on fuel dilution highlight that bioethanol has higher final boiling point and latent heat of vaporization. Both of these properties increase the tendency of the fuel to reach the crankcase and therefore increase the rate of fuel addition to the lubricant.86 In addition, the presence of bioethanol affects the lubricant in the ring pack in cold-start, warm-up and short-trip conditions due to its higher heat of evaporation compared to gasoline. One study was focused on investigating the accelerated oil dilution by using an engine test bench, and the results showed that fuel with low viscosity, density, surface tension and high front-end volatility is correlated to the fuel addition rate rather than the droplet size of the fuel spray and in-cylinder droplet evaporation rate.86 The distillation temperature and penetrating and solvency properties also have a significant effect on the fuel dilution in the long term.90 Fuel dilution may also be caused by leaking fuel injectors, excessive idle time, incomplete combustion, cold engine operating conditions, frequent short-trip driving and worn piston ring or excessive blow-by. Hence, the mode of injection (i.e. pre-injection and post-injection), late injection and fuel properties are the factors which influence the fuel dilution rate of lubricants.
As seen in the above literature, fuel properties also contribute to fuel dilution which is one of the main causes of lubricating oil degradation. During cold condition, engine fueled with bioethanol will encounter significant starting problem because of its high boiling point. If the temperature of lubricant does not reach operating temperature of 70 °C or over, bioethanol existing in the sump will not evaporate. Hence, based on this literature, the study on the optimization of fuel blends such as gasoline–ethanol and diesel–biodiesel blends at different blending concentrations should be encouraged to include the investigation into fuel properties like boiling point, heat of vaporization, distillation temperature, and penetrating and solvency properties because they are factors influencing the increase in fuel dilution rates of engine oil.
4.2. Fuel dilution rate of lubricants
A high level of fuel dilution in the lubricant indicates that there is serious contamination of the lubricant. It is known that fuel dilution degrades the quality of the lubricant and it is worth noting that it is crucial to maintain the quality of the lubricant in order to ensure optimum lubrication performance during engine operations. The design limit for fuel dilution is typically less than 5 wt% and a higher level of fuel dilution is unacceptable because this results in a significant reduction in the oil viscosity as well as the concentration of the oil additive. This in turn reduces the film thickness of the lubricant.85A number of researchers have investigated the effect of fuel dilution rate on the properties of automotive lubricants. One study showed that the properties of mineral oils degrade significantly at 1% fuel dilution, and the wear protection properties of these oils were totally deteriorated at 7% fuel dilution. In contrast, the stability resistance of synthetic oils improves at 1% fuel dilution but these oils lose almost all of their lubrication properties at 7% fuel dilution.93 Other researchers discovered that the maximum fuel dilution is 4% for gasoline engine, whereas 5% fuel dilution is sufficient to decrease the viscosity and flash point of the oil considerably and weakens the film stability of the oil.94 There are two mechanisms which influence the level of fuel dilution, the rate of fuel leaving the oil and the rate of fuel entering the oil. This occurs when the fuel fails to vaporize and remains in the cylinder in liquid form. The rate of evaporation from the oil plays a critical role in determining the overall dilution rate. It is important to determine the level of fuel dilution and keep it less than 5% to ensure that the lubricant remains effective.
4.3. Chemical reaction of fuels and lubricants in gasoline engines
Chemical reactions of fuels (bioethanol/gasoline) occur in the internal combustion engine due to the physicochemical properties of the fuels. Bioethanol is more chemically reactive than gasoline due to the presence of oxygen in the molecular structure of bioethanol. Hence, when bioethanol is added into gasoline, it will react more actively with oxygen during the combustion process and form unfavourable organic compounds in the gasoline engine.95 Moreover, since automotive lubricants consist of a base fluid and a variety of dissolved additives such as detergents and dispersants, it is possible that bioethanol–gasoline fuel dilution will affect the lubricant due to the chemical reactions with the base oil or additives.96 These chemical reactions will affect the combustion process, engine power, fuel consumption, lubricant properties, friction and wear, formation of engine deposits and oil drain intervals as well as the composition of the exhaust gases.97 Fig. 7 shows the harmful effects due to the chemical reactions of the fuel and lubricant. | ||
| Fig. 7 Harmful effects due to the chemical reactions of the fuel and engine oil. Credit taken from J. Hancsók, University of Pannonia, Hungary.97 | ||
 | ||
| Fig. 8 Flow chart showing the formation of sludge and varnish. Reproduced with permission from ref. 98, copyright 2009, Taylor and Francis. | ||
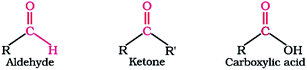 | ||
| Fig. 9 The formulation of aldehydes, ketones, carboxylic acids.99 | ||
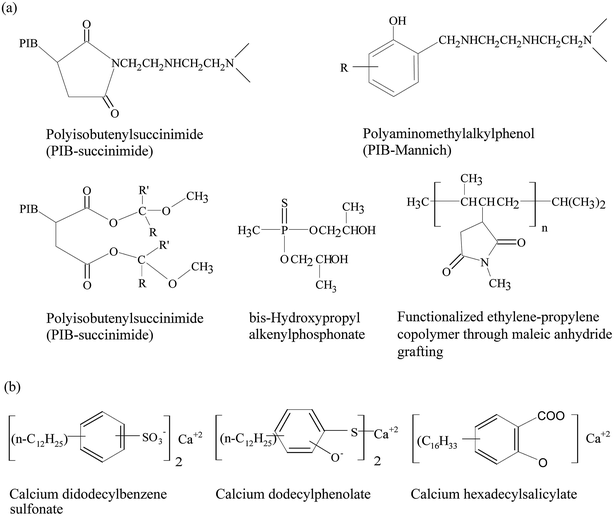 | ||
| Fig. 10 Chemical structures of: (a) dispersant molecules and (b) detergent molecules in lubricants. Reproduced with permission from ref. 98, copyright 2009, Taylor and Francis. | ||
The reaction of the fuel–oil mixture leads to the formation of resins, which are the complex mixtures of polar materials with high molecular weight containing nitrohydroxyl and carbonyl functional groups as well as sludge. The reaction involves the interaction between fuel and fuel-derived unsaturated hydrocarbons such as olefins and diolefins (aromatic compounds in gasoline) with NOX and oxygen, forming a class of compounds called nitro-nitrates.98,100 Olefins are known to form heavier deposits compared to other types of fuel hydrocarbons. Deposits form during oxidation and condensation, whereby NOX act as radical initiators. However, the structure and elemental composition of the polymeric deposits show that carbonyl compounds having low molecular weight in the blow-by are more readily included in their formation than nitrated intermediates. The crankcase oil is indeed a medium of reaction for the blow-by components. Even though the oxidation-stable synthetic oils help minimize the formation of varnish, the esters accumulate more pentane-insoluble than polyalphaolefins or alkylbenzenes.101 Polymerization involves an aldo-type condensation reaction, by which carbonyl compounds react to form polymeric species of various sizes.98
Fig. 11 shows the formation of oxidative compounds during oxidation of mineral oils. These oxidized products can polymerize and form insoluble resins on various engine components such as filter valves, pistons and rings. This affects adhesion as well as tolerance of the engine components. At a certain point, the resinous materials will interact with water, strong acids and unburned fuel containing high olefin and oxygenates to form water-in-oil emulsions or sludge. Fig. 12 shows the chemical reactions that take place during oxidative degradation of lubricant base oils. It shall be noted that the oil flow throughout the fuel system will be affected if the sludge is not dispersed properly, and this will lead to engine catastrophes.98 The chemical reactions that take place during the initiation, propagation and termination stages are described briefly as follows:102
 | ||
| Fig. 11 Formation of oxidative compounds during oxidation of mineral oils. Credit taken from Per Wiklund (NSP Research).103 | ||
 | ||
| Fig. 12 Chemical reactions that take place during oxidative degradation of lubricant base oils. Credit taken from Laura Corvinos Ancho (B.Eng. Chemical Engineering, C.C.JENSEN A/S).102 | ||
4.3.2.1 Initiation stage. In this stage, the hydrocarbons react with oxygen to form hydrocarbon free radicals. This reaction is catalysed by traces of transition metal ions such as copper, iron cobalt and chromium.
4.3.2.2 Propagation stage. In this stage, the hydrocarbon free radicals react with oxygen to form peroxide radicals which are highly reactive. This leads to further reaction with the hydrocarbons in the lubricant. This reaction leads to hydroperoxides and more hydrocarbon free radicals.
4.3.2.3 Termination stage. The reaction that takes place in this stage causes the hydroperoxides to separate and form oxygenated compounds such as aldehydes, ketones, alcohols and water. These compounds react further to form organic acids and polymeric products with high molecular weight. Further polycondensation and polymerization of these products lead to insoluble product known as sludge, which will precipitate as a thin film to form lacquer or varnish deposits on hot or cold metal surfaces.
5. Effects of bioethanol dilution on the physicochemical properties and performance of automotive lubricants
Even though bioethanol is widely accepted as an alternative fuel for gasoline engines, there are still issues concerning the effect of bioethanol dilution on the tribological properties of automotive lubricants which need to be addressed. In general, bioethanol dilution decreases the viscosity and TBN of the lubricant while it increases the TAN of the lubricant. Bioethanol dilution is highly undesirable since it leads to the oxidation of lubricant as well as the corrosion of engine components. The accumulation of bioethanol in the lubricant reduces the performance of the lubricant to protect the engine components against friction and wear. In addition, the presence of water in the chemical structure of bioethanol has a corrosive effect on fuel systems. The presence of water also leads to phase separation which has an adverse effect on engine operations.20 For this reason, it is important to highlight the effects of bioethanol dilution on the physicochemical properties and performance of automotive lubricants, which is the focus of this section.5.1. Effects of bioethanol dilution on the physicochemical properties
More importantly, the viscosity of the lubricant is greatly affected when it is contaminated by bioethanol. It was shown in a previous study that a low concentration of bioethanol decreases the viscosity of both base and formulated oils.105 Another study was conducted to investigate the effects of ethanol contamination on the properties of engine oils, in which 2 and 5% hydrate and anhydrous ethanol was added into base and formulated oils. The results showed that the addition of a low concentration of ethanol into the oil decreases the viscosity of the base and formulated oils, particularly for anhydrous ethanol.96 Veselá et al.106 conducted a study to compare the effect of BA95 fossil fuel and E85 on the quality and cleanliness of the engine oil at different temperatures. The tests were carried out on Saab 95 and 93 car engines lubricated with pure Mobil 1 0W-40 engine oil, and the properties were measured at 40 and 100 °C. The measurements were taken after a mileage of 7000 km, and the results showed that the viscosity of the engine oil decreases up to 20% for the Saab 95 car engine fuelled with E85, whereas the viscosity of the engine oil decreases more than 20% for the Saab 93 car engine. The viscosity measured at 40 °C is 69.379 and 69.263 mm2 s−1, respectively, whereas the viscosity for pure engine oil is 75.127 mm2 s−1. It shall be noted that the viscosity of the engine oil for both car engines fuelled with E85 could not be measured at 100 °C. In contrast, the viscosity of the engine oil was measurable at both temperatures when fossil fuel BA95 (octane value: 95) was used to fuel the engine, and the results showed that the viscosity of the engine oil for both car engines fuelled with this BA95 fossil fuel do not differ significantly from the viscosity of the pure engine oil at 40 and 100 °C. The results indicated that there is a significant reduction in the viscosity of the engine oil for the Saab 95 and 93 car engines fuelled with E85, which leads to a decrease in the lubricity of the engine oil. This in turn leads to a serious deterioration in the engine components, and moreover, the engine oil needs to be replaced on a frequent basis.
Tippayawong and Sooksarn107 evaluated the degradation of various types of lubricant used in small motorcycle engines. Four motorcycle engines were used in their experiments – one for each fuel-lubricant: (1) gasoline and mineral oil, (2) gasoline and synthetic-based oil, (3) 5% ethanol fuel and mineral oil and (4) 5% ethanol fuel and synthetic-based oil. The experiments were conducted over a period of 4–6 months and the mileage range was 1500–3000 km. They discovered that the viscosity of all lubricants decreases with the operating time (measured at distance level). The viscosity decreases by 20 and 45% for the synthetic-based oil and mineral oil, respectively, at a mileage of 3000 km. However, there is no significant difference between the effect of gasoline and 5% ethanol fuel on viscosity.
The viscosity of the lubricant plays a vital role in the formation of the lubricating film and rate of fuel consumption as well as heat generation in the pistons, rings, bearings and other engine components resulting from fluid friction. Previous studies have also shown that the engine operating conditions have a significant effect on the physicochemical properties of the lubricant. For instance, Hu et al.92 discovered that higher engine torque at the rated-power has a pronounced effect on the oil viscosity. They investigated the dilution of engine crankcase oil on the fuel injection of a turbocharged gasoline direct-injection (TGDI) engine, whereby they increased the high-speed end torque of the highly-boosted TGDI engine in order to enhance the acceleration performance. However, the results showed that the high-speed end torque increases fuel dilution up to 9% which significantly decreases the oil viscosity. Consequently, the engine components deteriorate significantly due to the decrease in oil lubricity. However, one study obtained contradictory results, whereby the viscosity of the engine oil actually increases when the engine was fuelled with ethanol in cold driving conditions. Jakóbiec and Mazanek100 found that the viscosity of the engine oil increases during winter driving conditions. They used SL/CF SAE 5W-30 engine oil to lubricate a motor vehicle engine fuelled with different fuels: (1) gasoline 95 and (2) gasoline with 5% (v/v) ethanol additive. The testing mileage range was 5000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km. The results revealed that there is only a slight change in the viscosity of the engine oil when the engine is fuelled with gasoline and 5% ethanol additive at a mileage of 5000 km. However, the viscosity of the engine oil increases significantly by 39% after a mileage of 30
000 km. The results revealed that there is only a slight change in the viscosity of the engine oil when the engine is fuelled with gasoline and 5% ethanol additive at a mileage of 5000 km. However, the viscosity of the engine oil increases significantly by 39% after a mileage of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km. They believed that the increase in oil viscosity is due to the presence of solid contaminants. Generally, in most conditions, it is apparent that the viscosity of the engine oil decreases when ethanol is blended with the gasoline fuel.
000 km. They believed that the increase in oil viscosity is due to the presence of solid contaminants. Generally, in most conditions, it is apparent that the viscosity of the engine oil decreases when ethanol is blended with the gasoline fuel.
Jakóbiec and Mazanek100 examined the changes in the TBN and TAN of SL/CF SAE 5W-30 engine oil. The experiments were carried out using a motor vehicle engine fuelled with two types of fuel: (1) gasoline 95 and (2) gasoline with 5% (v/v) ethanol additive. The testing mileage was within a range of 5000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km. The results showed that there is a significant decrease in the TBN of the engine oil for engine fuelled with gasoline blended with 5% ethanol additive, with a value of approximately 52% in winter driving conditions. In contrast, the TBN of the engine oil decreases by 41% for the engine fuelled with gasoline 95. Hence, it is evident that the decrease in the TBN is more pronounced for the engine fuelled with the bioethanol blend. The results also showed that the TAN of the engine oil is higher for the engine fuelled with gasoline blended with 5% ethanol additive. The increase in the TAN of the engine oil results in a serious deterioration in the engine components due to oil oxidation as well as corrosion. Other researchers have also discovered that the oxidation level is higher for ethanol fuel, which increases the TAN of the engine oil.68,108 Table 12 shows the results obtained by Jakóbiec and Mazanek,100 i.e. the variations in the TBN and TAN of the SL/CF SAE 5W-30 engine oil for two types of fuel (i.e. gasoline 95 and gasoline with 5% ethanol additive) in winter driving conditions.
000 km. The results showed that there is a significant decrease in the TBN of the engine oil for engine fuelled with gasoline blended with 5% ethanol additive, with a value of approximately 52% in winter driving conditions. In contrast, the TBN of the engine oil decreases by 41% for the engine fuelled with gasoline 95. Hence, it is evident that the decrease in the TBN is more pronounced for the engine fuelled with the bioethanol blend. The results also showed that the TAN of the engine oil is higher for the engine fuelled with gasoline blended with 5% ethanol additive. The increase in the TAN of the engine oil results in a serious deterioration in the engine components due to oil oxidation as well as corrosion. Other researchers have also discovered that the oxidation level is higher for ethanol fuel, which increases the TAN of the engine oil.68,108 Table 12 shows the results obtained by Jakóbiec and Mazanek,100 i.e. the variations in the TBN and TAN of the SL/CF SAE 5W-30 engine oil for two types of fuel (i.e. gasoline 95 and gasoline with 5% ethanol additive) in winter driving conditions.
| Property | Mileage (km) | Fuel | |||||
|---|---|---|---|---|---|---|---|
| 5000 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| Total acid number (mg KOH per g) | 3.91 | 4.83 | 6.12 | 6.63 | 7.94 | 9.10 | Gasoline 95 |
| 3.79 | 4.84 | 6.42 | 6.87 | 7.66 | 8.72 | Gasoline + 5% ethanol additive | |
| Total base number (mg KOH per g) | 10.04 | 9.65 | 8.88 | 8.25 | 7.06 | 6.10 | Gasoline 95 |
| 9.83 | 8.03 | 7.20 | 6.64 | 6.00 | 5.54 | Gasoline + 5% ethanol additive | |
5.2. Effect of bioethanol dilution on lubricant performance
The performance of automotive lubricants is influenced by the presence of contaminants such as soot, water and worn metal particles as well as acidic by-products of fuels. The presence of these contaminants alters the tribological properties of lubricants, which leads to wear and corrosion of the engine components. This is typically the case for flex-fuel vehicles which are usually fuelled with bioethanol. The effects of bioethanol dilution on friction and wear characteristics as well as sludge formation are discussed in the following sub-sections.In general, there are two ways to reduce friction between engine components: (1) using a lubricant with low viscosity or (2) adding friction modifiers into the lubricant.110 For example, molybdenum-containing additives are friction modifiers that are able to reduce the coefficient of friction to the range of 0.05–0.08. Even though molybdenum dialkyl dithiocarbamate (MoDTC) is one of the common friction modifiers available in the market, its lubrication capability degrades significantly in severe engine conditions (i.e. high temperature and high shear stresses as well as chemical attacks by fuel combustion products).111,112
However, it has been shown in previous studies that there is a reduction in the coefficient of friction due to bioethanol dilution, which is associated with the reduction in oil viscosity. One such study is the work of Ajayi et al.113 who investigated the effect of fuel dilution rate on the lubrication capability of lubricants exposed to three types of fuels: (1) pure gasoline (E0), (2) gasoline blended with 10% ethanol (E10) and (3) gasoline blended with 16% isobutanol (iB16). The tests were carried out using a marine engine which was operated in on-water conditions. A start-and-stop protocol was used for the tests. The results showed that the level of fuel dilution increases with an increase in the engine speed. The highest level of fuel dilution was observed for iB16, whereas the lowest level of fuel dilution was observed for E10. The results also showed that the high level of fuel dilution decreases the oil viscosity substantially regardless of the fuel used. The decrease in oil viscosity enhances the lubrication capability of the lubricant since it decreases friction.
The friction characteristics of automotive lubricants contaminated with ethanol and water have also been investigated in previous studies. De Silva114 investigated the effect of ethanol and water contamination on the friction characteristics of the lubricant for both piston rings and cylinder liner in cold-start, warm-up and short-trip conditions. Interestingly, the tribometer measurements showed that there is a significant reduction in friction when the contact surface between the piston rings and cylinder liner is lubricated with the lubricant contaminated with ethanol and water compared to the formulated oil. Likewise, Priest115 also observed a reduction in friction of the piston rings when the lubricant is contaminated with ethanol and water. He prepared four types of engine oil samples: (1) engine oil mixed with 5 wt% ethanol and 8 wt% water, (2) engine oil mixed with 5 wt% ethanol and 16 wt% water, (3) engine oil mixed with 10 wt% ethanol and 8 wt% water and (4) engine oil mixed with 10 wt% ethanol and 16 wt% water. The tests were carried out at the following test conditions: (1) temperature: 20 and 40 °C, (2) engine speed: 1500–2000 rpm and (3) engine load: 100 and 200 N. The results showed that there is a significant reduction in friction for the contact surfaces lubricated with lubricant–ethanol–water mixtures compared to the pure engine oil. The addition of ethanol and water into the lubricant improves the ability of the lubricant to reduce friction throughout the tested period.
Costa and Spikes96 compared the friction characteristics of formulated oils (the engine oils contaminated with hydrous and anhydrous ethanol and water) with those of base oil. The friction test results revealed that the addition of a low concentration of ethanol into the base oil slightly reduces friction at low engine speeds. In contrast, the addition of a low concentration of ethanol into the formulated oils reduces friction at high engine speeds. The effect of ethanol contamination on friction is similar for the base and formulated oils. The friction increases for the formulated oils at low engine speeds since the ethanol interferes with the formation of a thick boundary film. This leads to a reduction in the oil's film thickness from ca. 9 to ca. 2–3 nm.
However, it was shown that there is no reduction in friction when the lubricant is contaminated with bioethanol in an artificial alteration process where temperature and oxidation alter the properties of the lubricant. Lenauer et al.116 investigated the effect of bioethanol dilution on the friction characteristics of the lubricant using a ring cylinder system. They carried out tribological tests by setting up a model tribometer, followed by measuring the friction and wear characteristics of the piston ring. Two types of lubricant were used in the experiments: (1) mineral base oil (SAE 15W-40) and (2) base oil with acetic acid additive (SAE 5W-30). The results showed that the coefficient of friction remains constant throughout the duration of the experiments, and the difference in the coefficient of friction between the two lubricants is not significant. The coefficient of friction is low for the base oil, whereas the coefficient of friction is higher for the base oil with acetic acid additive. However, the authors did not provide any possible explanations for the higher coefficient of friction for the latter lubricant.
Based on the results discussed in this section, it can be deduced that friction is reduced for lubricants contaminated with bioethanol compared to pristine lubricants.
In general, wear and sludge are undesirable since they reduce the lubrication properties of the lubricant, which in turn reduces the lifespan of engine components. Wear and sludge also results in frequent oil drain intervals since the user needs to change the lubricant regularly. It was shown in a study that the use of E85 increases the formation of engine deposits in the gasoline engine compared to neat gasoline.32 A number of researchers have also investigated the effects of bioethanol dilution on wear characteristics and sludge formation in engines. Ajayi et al.113 investigated the effect of fuel dilution rate of three lubricants exposed to E0, E10 and iB16 fuels on friction and wear behaviour. The tests were carried out in the boundary lubrication regime. The results revealed that the dilution of these fuels have a significant effect on wear behaviour. Another study showed that engine wear increases during warm weather. Chui and Baker117 studied the effect of bioethanol dilution on the wear and corrosion characteristics of engine oil during warm-up driving conditions. The tests were conducted in accordance with the ASTM Sequence V-D gasoline engine test within a temperature range of 27–49 °C. The results showed that the concentration of bioethanol in the crankcase oil increases from 0 to 25%, whereas the concentration of iron in the crankcase oil increases from 200 to 2600 ppm after 192 operating hours. Another study showed that blending low concentrations of ethanol with gasoline has an insignificant effect on the degradation of the engine oil but it results in the formation of sludge. Besser et al.3 developed a novel laboratory-based artificial ageing method to investigate the effect of fuel dilution on oil degradation and formation of sludge and engine deposits. They formulated three types of engine oils: (1) engine oil mixed with bioethanol, (2) engine oil mixed with acetaldehyde and (3) engine oil mixed with acetic acid. The results showed that the addition of ethanol and acetaldehyde in the engine oil at low concentrations has a negligible effect on the progression of oil degradation. However, the results showed the presence of engine deposits at low concentrations of ethanol and the presence of some particles at high concentrations of ethanol. More importantly, the results showed the formation of heavy sludge for the engine oil mixed with acetic acid. It shall be highlighted here that the formation of sludge and engine deposits is undesirable since these materials will clog valves and orifices. In addition, it is an arduous task to dissolve sludge and engine deposits even at high temperatures. These materials also accelerate engine wear by trapping hard metal contaminants.102
However, wear and sludge can be reduced significantly by the incorporation of lubricant additives which help prevent auto-oxidation of the engine oil and minimize corrosion on the engine components. Several researchers have investigated the effect of bioethanol dilution on the performance of lubricants mixed with additives. It was shown that fuel blends containing 10 and 15% dry ethanol do not result in abnormal wear in engines.118,119 Costa and Spikes105 investigated the effect of ethanol dilution on the growth and stability of anti-wear tribofilms for lubricants containing zinc dialkyl dithiophosphate (ZDDP) additives. The results showed that the presence of ethanol fuel in the lubricants at 100 °C has no effect on the formation of anti-wear tribofilms. However, it shall be noted that the ethanol fully evaporates from the lubricants at this temperature. Lenauer et al.116 investigated the effect of bioethanol dilution on the friction and wear characteristics of lubricants used to lubricate a piston ring-cylinder liner system. The tribological tests were carried out by setting up a model tribometer to measure the friction and wear of the piston ring. Two types of lubricants were used in their study: (1) mineral base oil (SAE 15W-40) and (2) base oil with additive (SAE 5W-30). The results showed that the level of wear is lower for the mineral base oil compared to that for the base oil with additive in the presence of bioethanol. However, they concluded that both of these lubricants result in lower steady-state wear and thinner tribofilms compared to their pristine versions (i.e. lubricant without the presence of bioethanol).
Based on the studies reviewed in this section, it can be deduced that the level of wear and sludge increases when the lubricants are contaminated with bioethanol, especially those without additives.
6. Conclusion
The physicochemical properties of bioethanol and its blends, the various types of automotive lubricants commercially available in the market, bioethanol dilution and the key works pertaining to the effects of bioethanol dilution on the physicochemical properties and friction and wear characteristics of automotive lubricants as well as sludge formation are reviewed in this paper. The following conclusions can be drawn based on the works reviewed in this paper:• Bioethanol and its blends are promising alternative fuels since they can be derived from a variety of renewable feedstocks such as edible and non-edible feedstocks as well as algal biomass, and they produce less carbon oxide emissions compared to fossil fuels such as gasoline and diesel.
• Bioethanol dilution is the condition in which unburned bioethanol accumulates in the crankcase through the cylinder walls, which in turn contaminates the lubricant. The mode of injection (pre-injection and post-injection) and the fuel properties are the causes of bioethanol dilution. Bioethanol dilution is higher compared to gasoline because of its higher heat of vaporization and boiling point. The interaction between bioethanol and lubricant leads to oxidative degradation in the lubricant, which alters the lubricant's physicochemical properties as well as friction and wear characteristics.
• Bioethanol contains high oxygen content and has a hygroscopic nature which attracts the amount of water in lubricants. It is possible that the complex mixture of bioethanol, water and lubricant as well as lubricant additives interact with each other over a period of time especially at high temperature, resulting in highly oxidative degradation in lubricants, sludge formation and wear of engine components.
• In most cases, the viscosity of the lubricant decreases when the lubricant is contaminated with bioethanol. However, there are cases whereby the viscosity of the lubricant increases due to the formation of oxidative products such as polymeric compounds and sludge.
• The total base number decreases while the total acid number of the lubricant increases with an increase in the bioethanol dilution rate. This results in the oxidation of lubricant. The decrease in the total base number and the increase in the total acid number are detrimental to engine components since the lubricant loses its ability to protect these components from corrosion.
• Bioethanol dilution has a significant effect on the formation of engine deposits and sludge. This is due to the fact that bioethanol has higher oxygen and water content which promotes oxidation of the lubricant. The lubricant loses its lubrication properties, resulting in the formation of engine deposits and sludge. Bioethanol helps in reducing friction, provided that it is blended with gasoline or diesel. This may be due to the fact that there is the enhancement in the lubricity of bioethanol–gasoline blends and bioethanol–diesel blends, which in turn help reduce the friction of engine components when lubricant is diluted with either one of these blends. It has been shown in previous studies that the coefficient of friction decreases due to the decrease in the viscosity of the lubricant as a result of bioethanol dilution.
• The incorporation of lubricant additives such as detergents, dispersants, friction modifiers, anti-wear and antioxidant additives helps prevent the degradative oxidation of lubricant and minimize the corrosion of engine components. These additives also help reduce friction and wear of the engine components.
Owing to the importance of bioethanol dilution, it is recommended that future studies should be carried out to explore the effects of bioethanol blends (at various concentrations of bioethanol) on the physicochemical properties, friction and wear characteristics of automotive lubricants in various engine operating conditions. This is indeed an area worthy of investigation, and the findings will be useful to develop new lubricants with superior properties that are compatible with bioethanol blends.
Acknowledgements
The first author is greatly indebted to the Japan International Cooperation Agency (JICA) and AUN/Seed-Net for funding the author's postgraduate study and research grant under the ‘Collaborative Research’ programme. The authors would like to thank the Ministry of Higher Education and University of Malaya, Malaysia for the financial assistance through High Impact Research Grant titled: Development of alternative and renewable energy carrier (DAREC) with Grant Number UM.C/HIR/MOHE/ENG/60.References
- M. Balat, Energy Convers. Manage., 2011, 52, 858–875 CrossRef CAS.
- M. J. Bradshaw, Geogr. J., 2010, 176, 275–290 CrossRef.
- C. Besser, C. Schneidhofer, N. Dörr, F. Novotny-Farkas and G. Allmaier, Tribol. Int., 2012, 46, 174–182 CrossRef CAS.
- E. Martinot, Renewables 2005: Global status report, Worldwatch Institute, Washington, DC, 2005 Search PubMed.
- Alternative motor fuels act of 1988, http://www.afdc.energy.gov/pdfs/3143.pdf, accessed 12 January, 2016.
- L. B. Carlos Bastian-Pinto and M. de Lemos Alves, Annals of Operations Research, 2010, 176, 333–348 CrossRef.
- M. R. S. Dabrina, D. Dutcher, S. L. Thompson, J. M. Medrano, D. S. Gross, D. B. Kittelson and P. H. McMurry, Atmosphere, 2011, 2, 182–200 CrossRef.
- M. Matti Maricq, Combust. Flame, 2012, 159, 170–180 CrossRef CAS.
- M. Cavalcanti, A. Szklo and G. Machado, Renewable Energy, 2012, 43, 423–433 CrossRef.
- R. Linde, Emission and experience with E85 converted cars in the BEST project, Sweden, Vaxjo, 2010 Search PubMed.
- L. Pelkmans, G. Lenaers, J. Bruyninx, K. Scheepers and I. De Vlieger, Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automobile Engineering, 2011, 0954407011407254 Search PubMed.
- R. Magnusson, C. Nilsson and B. Andersson, Environ. Sci. Technol., 2002, 36, 1656–1664 CrossRef CAS PubMed.
- A. Ener and Y. Devel, Renewable Energy Developments and Potential in the Greater Mekong Subregion, Mandaluyong, The Philippines, 2015 Search PubMed.
- A. O. Akponah and E. M. Ubogu, Eur. J. Exp. Biol., 2013, 3, 247–253 Search PubMed.
- R. M. Kevin Fisher, Trimeric Corporation, 2006.
- D. R. Lide, CRC handbook of chemistry and physics, CRC Press, Maryland, 81st edn, 2000 Search PubMed.
- M. Windholz, An encyclopedia of chemicals, drugs, and biologicals, Merck, Rahway (N.J.), 9th edn, 1979 Search PubMed.
- M. L. Randazzo and J. R. Sodré, Fuel, 2011, 90, 98–103 CrossRef CAS.
- A. Tandon, A. Kumar, P. Mondal, P. Vijay, U. Bhangale and D. Tyagi, Br. J. Environ. Clim. Change, 2011, 1, 28–43 CrossRef PubMed.
- H. Hazar and İ. Temizer, The engine performance of a diesel engine and the research of the effect of fuel additives on engine oil and engine parts, 2012 Search PubMed.
- T. C. K. Owen, Automotive fuels handbook, Society of Automotive Engineer, Warrendale, PA (USA), 1990 Search PubMed.
- D.-g. Li, H. Zhen, L. Xingcai, Z. Wu-gao and Y. Jian-Guang, Renewable Energy, 2005, 30, 967–976 CrossRef CAS.
- H. Jafari, M. Idris, A. Ourdjini, H. Rahimi and B. Ghobadian, Mater. Corros., 2010, 61, 432–440 CAS.
- L. Baena, M. Gómez and J. Calderón, Fuel, 2012, 95, 320–328 CrossRef CAS.
- M. Boons, R. Van den Bulk and T. King, The impact of E85 use on lubricant performance, Report 0148–7191, SAE Technical Paper, 2008 Search PubMed.
- S. E. Schwartz, D. J. Smolenski and S. L. Clark, Entry and retention of methanol fuel in engine oil, SAE Technical Paper, 1988 Search PubMed.
- M. Lapuerta, O. Armas and J. M. Herreros, Fuel, 2008, 87, 25–31 CrossRef CAS.
- D. Ljubas, H. Krpan and I. Matanović, Nafta, 2010, 61, 73–79 CAS.
- A. Dhar and A. K. Agarwal, Fuel, 2014, 130, 112–119 CrossRef CAS.
- S. George, S. Balla, V. Gautam and M. Gautam, Tribol. Int., 2007, 40, 809–818 CrossRef CAS.
- M. Balat and H. Balat, Appl. Energy, 2009, 86, 2273–2282 CrossRef CAS.
- C. Wyman, Handbook on bioethanol: production and utilization, CRC press, 1996 Search PubMed.
- REBEL WP7: Bioenergy, http://www.responsiblebusiness.eu/display/rebwp7/PDF, accessed 16 January, 2016.
- A. Avinash and P. Sasikumar, Alexandria Eng. J., 2016, 55, 351–356 CrossRef.
- A. C. Hansen, Q. Zhang and P. W. Lyne, Bioresour. Technol., 2005, 96, 277–285 CrossRef CAS PubMed.
- H. S. Yücesu, T. Topgül, C. Cinar and M. Okur, Appl. Therm. Eng., 2006, 26, 2272–2278 CrossRef.
- E. Torres-Jimenez, M. S. Jerman, A. Gregorc, I. Lisec, M. P. Dorado and B. Kegl, Fuel, 2011, 90, 795–802 CrossRef CAS.
- P. Kwanchareon, A. Luengnaruemitchai and S. Jai-In, Fuel, 2007, 86, 1053–1061 CrossRef CAS.
- M. n. Lapuerta, R. García-Contreras, J. Campos-Fernández and M. P. Dorado, Energy Fuels, 2010, 24, 4497–4502 CrossRef CAS.
- U. Majumder, P. Chakraborti, R. Banerjee and B. Debbarma, Renewable Energy, 2016, 86, 972–984 CrossRef CAS.
- Bioethanol: Advantages and Disadvantages of Bioethanol, http://www.bioethanol-np.blogspot.my/p/advantages-of-bioethanol/html, accessed 23 December, 2015.
- A. M. Shetye, A. Ganguly, S. Simha and S. Acharjee, Experimental Investigation Of Tribological Properties Of Lubricating Oil For Biodiesel Fuelled Single Cylinder Diesel Engine, 2013 Search PubMed.
- S. Kim and B. E. Dale, Biomass Bioenergy, 2004, 26, 361–375 CrossRef.
- Bioethanol as a growth market, http://www.cropenergies.com/en/Bioethanol/Markt/Dynamisches_Wachstum/, accessed 16 February, 2016.
- G. Koçar and N. Civaş, Renewable Sustainable Energy Rev., 2013, 28, 900–916 CrossRef.
- A. Demirbas, Energy Sources, Part B, 2007, 2, 391–401 CrossRef CAS.
- Global Ethanol Production-Alternative Fuel Data Center, http://www.afdc.energy.gov/data/10331, accessed 16 February, 2016.
- E. Ajav and O. Akingbehin, A study of some fuel properties of local ethanol blended with diesel fuel, Agricultural Engineering International: CIGR Journal, 2002 Search PubMed.
- T. Letcher, South African Journal of Science, 1983, 79, 4–7 CAS.
- H. Aditiya, W. Chong, T. Mahlia, A. Sebayang, M. Berawi and H. Nur, Waste Manag., 2016, 47, 46–61 CrossRef CAS PubMed.
- F. Yüksel and B. Yüksel, Renewable Energy, 2004, 29, 1181–1191 CrossRef.
- S. H. Park, S. H. Yoon and C. S. Lee, Appl. Energy, 2014, 135, 286–298 CrossRef CAS.
- A. Matuszewska, M. Odziemkowska and J. Czarnocka, Properties of bioethanol-diesel oil mixtures, 2013 Search PubMed.
- S. H. Park, J. Cha and C. S. Lee, Appl. Energy, 2012, 99, 334–343 CrossRef CAS.
- G. Chen, Y. Shen, Q. Zhang, M. Yao, Z. Zheng and H. Liu, Energy, 2013, 54, 333–342 CrossRef CAS.
- M. N. Rahman, N. Atan, A. Mokhtar and A. Khalid, Influences of intake temperature and bio-petrol fuel temperature on SI engine: an overview, 2014 Search PubMed.
- M. E.-A. A. F. Kheiralla, M. Y. Hassan, M. A. Hussen and H. I. Osman, University Of Khartoum Engineering Journal, 2011, 1, 22–28 Search PubMed.
- R. N. Mehta, M. Chakraborty and P. A. Parikh, Indian J. Chem. Technol., 2012, 19, 134–139 CAS.
- E. Alptekin and M. Canakci, Fuel, 2009, 88, 75–80 CrossRef CAS.
- B. Xu, Y. Qi, W. Zhang and S. Cai, Int. J. Automot. Tech., 2007, 8, 9–18 Search PubMed.
- A. F. Kheiralla, University Of Khartoum Engineering Journal, 2011, 1, 22–28 Search PubMed.
- R. Reynolds, Changes in Diesel Fuel. The Service Technician's Guide to Compression Ignition Fuel Quality, National Biodiesel Board, Jefferson City, USA, 2007 Search PubMed.
- E. Christensen, J. Yanowitz, M. Ratcliff and R. L. McCormick, Energy Fuels, 2011, 25, 4723–4733 CrossRef CAS.
- C. Prakash, Report prepared for the Transportation Systems Branch, Air Pollution Prevention Directorate, Environment Canada, November, http://www.ec.gc.ca/transport/publications/ethgas/ethgastoc.htm, 1998.
- J. Bacha, J. Freel, A. Gibbs, L. Gibbs, G. Hemighaus, K. Hoekman, J. Horn, A. Gibbs, M. Ingham and L. Jossens, Chevron Global Marketing, 2007 Search PubMed.
- S. G. Inc., ASTM D975 Diesel Fuel Specification Test, Report 801-386-5007, Syntek Global Inc., Draper, 12382 South Gateway Park Place Suite B800, UT 84020 Search PubMed.
- M. Al-Hasan, Energy Convers. Manage., 2003, 44, 1547–1561 CrossRef CAS.
- M. N. A. M. Yusoff, N. W. M. Zulkifli, B. M. Masum and H. H. Masjuki, RSC Adv., 2015, 5, 100184–100211 RSC.
- Lubricant Additives Market Demand, https://www.globenewswire.com/news-release/2016/05/31/844569/0/en/Lubricant-Additives-Market-Demand-Will-Reach-5-36-Million-Tons-By-2024-Expected-To-Grow-At-2-5-CAGR-Grand-View-Research-Inc.html, accessed 30 May, 2016.
- A. Erdemir, Tribol. Int., 2005, 38, 249–256 CrossRef CAS.
- S. C. Tung and M. L. McMillan, Tribol. Int., 2004, 37, 517–536 CrossRef CAS.
- Global Analysis of the Synthetic and Bio-based Lubricants Market, http://www.persistencemarketresearch.com/article/global-synthetic-and-bio-based-lubricants-market.asp, accessed 31 May, 2016.
- Chemical Sector Research Lubricating Preparations lubricants, http://www.investingeorgia.org/en/ajax/downloadFile/640/Chemical_Sector_Research_-_Lubricating_Preparations, accessed 31 May, 2016.
- Global Lubricants Market Size, Share, Development, Growth and Demand Forecast to 2020 Industry, http://www.prnewswire.com/news-releases/global-lubricants-market-size-share-development-growth-and-demand-forecast-to-2020–industry-insights-by-product-mineral-synthetic-bio-based-by-application-industrial-automotive-grease-others-300218229.html, accessed 30 May, 2016.
- O. Smith, M. Priest, R. Taylor, R. Price and A. Cantley, World Tribology Congress III, American Society of Mechanical Engineers, 2005 Search PubMed.
- R. J. Prince, in Chemistry and Technology of Lubricants, London, New York, 3rd edn, 2010, ch. 1 Search PubMed.
- P. Nagendramma and S. Kaul, Renewable Sustainable Energy Rev., 2012, 16, 764–774 CrossRef CAS.
- R. M. Mortier, S. T. Orszulik and M. F. Fox, in Chemistry and technology of lubricants, Spinger, 3rd edn, 1992, ch. 2 Search PubMed.
- A. Jackson, presented in part at the International Tribology Conference 1987, Melbourne, 2-4 December 1987: Preprints of Papers, Institution of Engineers, Australia, 1987 Search PubMed.
- A. Ing, Biobased Lubricants: A Viability Study, Brisbane, Australia, 2009 Search PubMed.
- S. Arumugam and G. Sriram, Proc. Inst. Mech. Eng., Part J, 2012, 1350650112458398 Search PubMed.
- P. S. Lathi and B. Mattiasson, Appl. Catal., B, 2007, 69, 207–212 CrossRef CAS.
- Introducing the SAE 8 and SAE 12 viscosity grades, http://www.oilspecifications.org/articles/introducing-the-sae-8-and-sae-12-viscosity-grades.php, accessed 21 January, 2016.
- S. Shanta, G. Molina and V. Soloiu, Adv. Tribol., 2011, 2011, 820795 Search PubMed.
- G. X. Gu, Development and application of a lubricant composition model to study effects of oil transport, vaporization, fuel dilution, and soot contamination on lubricant rheology and engine friction, Massachusetts Institute of Technology, 2014 Search PubMed.
- M. Wattrus, Fuel Property Effects on Oil Dilution in Diesel Engines, SAE Technical Paper, 2013 Search PubMed.
- D. Uy, R. J. Zdrodowski, A. E. O'neill, S. J. Simko, A. K. Gangopadhyay, M. Morcos, F. Lauterwasser and G. Parsons, Tribol. Trans., 2011, 54, 749–763 CrossRef CAS.
- F. Gili, A. Igartua, R. Luther and M. Woydt, Lubr. Sci., 2011, 23, 313–330 CrossRef CAS.
- A. J. Martyr and M. A. Plint, in Engine testing: the design, building, modification and use of powertrain test facilities, Elsevier, 2012 Search PubMed.
- S. M. Shanta, Master, Investigations of the tribological effects of engine oil dilution by vegetable and animal fat feedstock biodiesel on selected surfaces, Georgia Southern University, 2011 Search PubMed.
- T. Sagawa, H. Fujimoto and K. Nakamura, Study of Fuel Dilution in Direct-Injection and Multipoint Injection Gasoline Engines, SAE Technical Paper, 2002 Search PubMed.
- T. Hu, H. Teng, X. Luo and B. Chen, SAE International Journal of Engines, 2015, 8, 1107–1116 Search PubMed.
- Y. A. Gur'yanov, Chem. Technol. Fuels Oils, 2007, 43, 30–36 CrossRef.
- J. Cerny and I. Vaclavickova, Goriva Maziva, 2006, 45, 323–330 Search PubMed.
- A. C. Santos, The Blend Ethanol/Gasoline and Emission of Gases, 2012 Search PubMed.
- H. L. Costa and H. Spikes, Tribol. Trans., 2015, 58, 158–168 CrossRef CAS.
- J. Hancsók, J. Auer, J. Baladincz, Z. Kocsis, L. Bartha, M. Bubálik and I. Molnár, Pet. Coal, 2005, 47, 55–64 Search PubMed.
- C. A. P. Tze-Chi Jao, in Handbook of detergents, part E: applications, ed. U. Zoller, CRC Press, 2008, vol. 141 Search PubMed.
- Aldehydes, Ketones and Carboxylic Acids, http://www.ncert.nic.in/ncerts/l/lech203.pdf, accessed 04 March 2016.
- J. Jakóbiec and A. Mazanek, Teka Komisji Motoryzacji i Energetyki Rolnictwa, 2009, 9, 99–113 Search PubMed.
- I. Spilners and J. Hedenburg, J. Am. Chem. Soc., 1981, 26, no. CONF-810308-(vol. 2) Search PubMed.
- L. C. Ancho, Oxidation in lubricant base oils, http://www.cjc.dk/fileadmin/user_upload/pdf/News_Press/Press_Info/Oxidation_in_lubricant_base_oils_The_Filter_2006.pdf, accessed 20 March 2016.
- Unravelling the chemistry of lubricant ageing, http://nynas.episerverhosting.com/Segment/Base-oils/Knowledge-Tank/Technical-articles/Unravelling-the-chemistry-of-lubricant-ageing/, accessed 20 March 2016.
- J. G. Wills, Lubrication Fundamentals, Marcel Decker, New York, Basel, 1980 Search PubMed.
- H. Spikes and H. Costa, Tribol. Int., 2015, 93, 364–376 Search PubMed.
- K. Veselá, M. Pexa and J. Marik, presented in part at International Scientific Conference, Agronomy Research, 2014, vol. 12, pp. 425–430 Search PubMed.
- N. Tippayawong and P. Sooksarn, Maejo Int. J. Sci. Technol., 2010, 4, 201–209 CAS.
- J. Friedl and U. Stimming, Electrochim. Acta, 2013, 101, 41–58 CrossRef CAS.
- G. Stachowiak and A. W. Batchelor, Engineering tribology, Elsevier, Butterworth-Heinemann, Oxford, 3rd edn, 2005 Search PubMed.
- M. De Feo, C. Minfray, M. D. B. Bouchet, B. Thiebaut, T. Le Mogne, B. Vacher and J. Martin, Tribol. Int., 2015, 92, 126–135 CrossRef CAS.
- C. Grossiord, K. Varlot, J.-M. Martin, T. Le Mogne, C. Esnouf and K. Inoue, Tribol. Int., 1998, 31, 737–743 CrossRef CAS.
- G. Spengler and A. Webber, Chem. Ber., 1939, 92, 2163–2171 CrossRef.
- O. O. Ajayi, C. Lorenzo-Martin, G. Fenske, J. Corlett, C. Murphy and S. Przesmitzki, J. Tribol., 2016, 138, 021603 CrossRef.
- P. R. De Silva, The impact of biofuel ethanol on lubrication and friction in the piston assembly of a gasoline engine, Diss, University of Leeds, 2012 Search PubMed.
- M. Priest, Impact of ethanol biofuel on automotive piston ring friction,https://www.institutes.engineering.leeds.ac.uk/thermofluids/research/tribology-surface-engineering/current-research/impact-of-ethanol-biofuel.shtml, accessed 28 March 2016.
- C. Lenauer, C. Tomastik, T. Wopelka and M. Jech, Tribol. Int., 2015, 82, 415–422 CrossRef CAS.
- G. K. Chui and R. E. Baker, The IV International Symposium on Alcohol Fuels Technology, Guaruja-SP-Brasil, 5–8 Oct. 1980, Instituto de Pesquisas Tecnologicas do Estado de Sao Paulo-IPT, Sao Paulo, Brasil, 1980 Search PubMed.
- A. C. Hansen, A. P. Vosloo, P. W. L. Lyne and P. Meiring, Agric. Eng. S. Afr., 1982, 16, 50–53 Search PubMed.
- I. Hashimoto, H. Nakashima, K. Komiyama and Y. Maeda, et al., SAE Technical Paper, 1982, DOI:10.4271/820497.
| This journal is © The Royal Society of Chemistry 2016 |