Nanomagnetic zirconia-based sulfonic acid (Fe3O4@ZrO2-Pr-SO3H): a new, efficient and recyclable solid acid catalyst for the protection of alcohols via HMDS under solvent free conditions
Azadeh Tadjarodi*,
Rahim Khodikar and
Hosssein Ghafuri
Department of Chemistry, Iran University of Science and Technology, Narmak, Tehran 16846-13114, Iran. E-mail: tajarodi@iust.ac.ir
First published on 28th June 2016
Abstract
In the present work, sulfonic acid functionalized nanomagnetic zirconia is prepared by the reaction of (3-mercaptopropyl)trimethoxysilane and nanomagnetic zirconia. Then, nanomagnetic zirconia-based sulfonic acid (Fe3O4@ZrO2-Pr-SO3H) is synthesized through direct oxidation of the thiol group by hydrogen peroxide and H2SO4 subsequently. The catalyst was characterized by Fourier transform infrared spectroscopy (FT-IR), field emission scanning electron microscopy (FE-SEM) imaging, energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD) measurements and vibrational sample magnetometry (VSM). It was used as an effective nanocatalyst with high catalytic activity for the protection of alcohols using hexamethyldisilazane (HMDS) under solvent-free conditions at room temperature. The solid nanocatalyst can easily be separated and reused several times without significant loss of its catalytic activity. Also, in addition to its being inexpensive and the simplicity of the separation process, this heterogeneous catalyst has shown a good chemoselectivity in the reactions.
Introduction
During the past decade, over 90% of organic reaction processes have employed catalysts. These catalysts play an important role in organic synthesis and transformations.1 Catalysts can be classified into two categories: homogenous2 and heterogeneous.3 Homogeneous acid catalysts such as HCl, H2SO4, HNO3, HClO4 and HF are considerably used in industrial processes but these catalysts have drawbacks in handling, producing toxic waste and corrosive properties. In recent years, there has been a growing interest in developing heterogeneous acid catalysts for organic reactions. Solid acid catalysts supply few opportunities for recovering and recycling catalysts from organic reactions. Also these catalysts are environmentally friendly.In this context, a heterogeneous catalyst based on nanoparticle (ZrO2) has been introduced, which is an inorganic material with chemical inertness. Also zirconia has a powerful resistance in comparison with alkalis and acids. Therefore, it can be used as suitable core for coating nanoparticle Fe3O4.4–7 Also in this work, magnetic zirconia functionalized sulfonic acid as heterogeneous solid acid catalyst used for carried various organic reactions.
The protection of alcohols is one of important and necessary process for multistep organic synthesis. Generally, protection of alcohols has been carried out by different reagents such as alkylsilanes8–10 and bis(trimethylsilyl)amine (HMDS)11 in the presence of a suitable catalyst.12 However, all above mentioned agents except HMDS suffered from serious problems such as toxicity, low yield of product, low selectivity, but HMDS are the useful and more popular compound for the protection of hydroxyl functional group by transforming silylether. Bis(trimethylsilyl)amine (HMDS), a cheap and stable reagent, can be used as an alternative silylating agent for preparation of silylethers from –OH alcohols compound.13,14 The main problem is the low silylating power and unsatisfactory yield.15 Therefore, a suitable catalyst in the reactions should be used.16 Lately, some catalysts used for the protection of alcohols reaction such as, (CH3)3SiCl,17 ZnCl4,18 LiClO4,19 MgBr2·OEt2,20 LaCl3,21 sulfonic acid-functionalized nanoporous.22 These catalysts are effective, however, they have some significant drawbacks such as poor silylating power, moisture-sensitive, long reaction time, wearisome workups, solvent usage and difficulty in the recovery of catalyst, severe reaction condition and high cost.23
Therefore, this protocol is, the development of not extreme and selective method for protection of alcohols that is done without solvent and in a short time and also has simple recovery by magnet, and environmentally friendly at room temperature (r.t).24 In this paper, the synthesis of chemically adsorbed sulfonic acid on Fe3O4@ZrO2 was obtained by the reaction post-synthesis (grafting)25 of (3-mercaptopropyl) trimethoxysilane and Fe3O4@ZrO2. Then oxidation of thiols by H2O2 and 1 mL H2SO4 successfully was carried out.26 Catalyst Fe3O4@ZrO2-Pr-SO3H as a new catalyst has high efficiency for organic reactions and also it is useful as catalyst having Brønsted acid properties for the preparation derivatives protection of alcohols. The characterization of Fe3O4@ZrO2-Pr-SO3H was performed with use of different techniques such as FT-IR, SEM, EDX, VSM and XRD.27
Experimental
Chemical materials were purchased from Merck Co. and utilized without further purification. FT-IR spectra were recorded as KBr pellets on a Shimadzu-8400 sin the range 400–4000 cm−1. The X-ray diffraction (XRD) pattern was recorded using a STOE powder diffractometer with Co kα, (λ = 1.789 Å) irradiation. Also for studying the morphology of catalyst scanning electron microscopy (SEM), entire image were taken by a Hitachi S4160 FESM. Magnetic characteristic of the particle was assessed with a vibrating sample magnetometer (VSM, lake shore 7410). The protection products were analyzed with a gas chromatograph (Shimadzu) equipped with a HP-5 capillary column.Preparation of Fe3O4@ZrO2 nanoparticle
Fe3O4 nanoparticles were synthesized utilizing Massart's procedure28 and then the core–shell Fe3O4@ZrO2 was prepared according to the applying method reported previously.29–33 Briefly, 0.2 g of Fe3O4 nanoparticles (NPs) homogenously were dispersed in HCl 0.1 M for 15 min in order to activate the nanoparticles surface. After magnetic particles were separated by magnet from aqueous solution. It was washed with distilled water two times. The nanoparticles again were dispersed in the mixture of 70 mL ethanol, 30 mL distilled water and 1 mL concentrated NH3 solution (28%). Finally TEOS (0.05 g, 2.4 mmol) was added to the mixture and stirred for 6 h at r.t. Afterward, silica coated Fe3O4 was separated by magnet and washed with ethanol and distilled water at least three times. Nanoparticles coated in the previous step, in the mixture of 0.4 g (1.1 mmol) CTAB (as surfactants), 70 mL distilled water, 2 mL NH3 and 70 mL ethanol for 30 min was stirred continuously until homogenized and uniform dispersion. Then zirconium nitrate, Zr(NO3)4 (0.7 g, 3.03 mmol), dissolved in the water was added drop-wise to the stirring mixture for 6 h at r.t. The final product was collected by magnet and washed with ethanol and distilled water. For removal of surfactant, the obtained particles were refluxed by acetone in the Soxhlet extractor for 32 h. Afterward, it was separated, washed by distilled water and dried at 80 °C to get Fe3O4@ZrO2.Preparation of 3-mercaptopropyl magnetic zirconia (MPMZ)
0.6 g of Fe3O4@ZrO2 was added to 4 mL dry toluene, 2 mL (10.77 mmol) of (3-mercaptopropyl) trimethoxysilane, and the reaction mixture was refluxed for 18 h. The nanoparticles were separated by magnet and washed with hot toluene for 12 h in the continuous extraction. Then it was dried for overnight at 110 °C to achieve surface bond thiol from Fe3O4@ZrO2-Pr-SH (MPMZ) group.Preparation of solid Fe3O4@ZrO2 based sulfonic acid
MPMZ was oxidized by 5 mL H2O2 and 1 mL H2SO4 in the 20 mL methanol for 12 h at r.t and washed three times with distilled water. In order to ensure complete protonation, the solid catalyst will be suspended in the H2SO4 solution 20% for 5 h. After that, the mixture was separated by magnet and washed three times with distilled water and acetone to get Fe3O4@ZrO2-Pr-SO3H catalyst (Scheme 2). | ||
| Scheme 1 The protection reaction of alcohol in the presence of synthesized magnetic acidic nanocatalyst (Fe3O4@ZrO2-Pr-SO3H) in room temperature and solvent free condition. | ||
FT-IR analysis
FT-IR spectra of Fe3O4, Fe3O4@ZrO2 and Fe3O4@ZrO2-Pr-SO3H samples are shown in Fig. 1. In Fig. 1a, there are the peaks at 1635 and 3452 cm−1, which related to –OH bending and stretching vibrations of the absorbed water molecules, respectively.There is the peak at 584 cm−1 that showing the presence of Fe–O. Also the peaks at 1054 and 630 cm−1 belong to vibrations Si–O and Zr–O, respectively (Fig. 1b). The vibrational peak of S–H is seen at 2559 cm−1 as shown in Fig. 1c. Because of weak dipole vibration of thiol bonds, observed peak is weak.34,35 The appeared peaks at 1045, 1089 and 1188 cm−1 were attributed to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric, symmetric and S–O vibrations of the (–SO3H) groups, respectively36,37 (Fig. 1d). In addition, the CH stretching vibrations were observed at 2842 and 2929 cm−1.
O asymmetric, symmetric and S–O vibrations of the (–SO3H) groups, respectively36,37 (Fig. 1d). In addition, the CH stretching vibrations were observed at 2842 and 2929 cm−1.
XRD patterns
X-ray diffraction patterns (XRD) for Fe3O4, Fe3O4@ZrO2 and Fe3O4@ZrO2-Pr-SO3H are given in Fig. 2. As well as XRD of the magnetic nanoparticles of Fe3O4 in the cubic phase is a good agreement with (PDF no. 01-075-0499) as shown in Fig. 2a. Also the XRD patterns of Fe3O4@ZrO2 and Fe3O4@ZrO2-Pr-SO3H are similar with XRD pattern for Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30,31 as given in Fig. 2b and c. It demonstrates that the modification has not considerable effect on the phase of Fe3O4. For this reason, ZrO2 is amorphous. Organic groups and nanoparticles available in amorphous structure have no effect in the crystal structure of compounds. Due to this fact, XRD patterns of Fe3O4@ZrO2 and Fe3O4@ZrO2-Pr-SO3H are similar.
30,31 as given in Fig. 2b and c. It demonstrates that the modification has not considerable effect on the phase of Fe3O4. For this reason, ZrO2 is amorphous. Organic groups and nanoparticles available in amorphous structure have no effect in the crystal structure of compounds. Due to this fact, XRD patterns of Fe3O4@ZrO2 and Fe3O4@ZrO2-Pr-SO3H are similar.
FE-SEM analysis
The FE-SEM images for the pure Fe3O4@ZrO2 and Fe3O4@ZrO2-Pr-SO3H are illustrated in Fig. 3. According to this chart, the particle size bare Fe3O4@ZrO2 distribution is narrow and the size of most of the particles is about 20–70 nm as given in (Fig. 4a) and (Fig. 3). Then, the powder is aggregated because of modification by sulphate and form strong hydrogen bonds between the –SO3H groups (Fig. 4b).EDX analysis for Fe3O4@ZrO2 and Fe3O4@ZrO2-Pr-SO3H
The EDX spectrum of Fe3O4@ZrO2 (Fig. 5a) displays the presence of iron, zirconium and oxygen elements. After modification of Fe3O4@ZrO2, in the EDX spectrum of Fe3O4@ZrO2-Pr-SO3H (Fig. 5b), some other elements such as sulphur and carbon were observed. The content of sulfur and carbon was 6.56 and 17.08 wt%, respectively. Also the reduction of iron peak intensity demonstrates that Fe3O4@ZrO2 coated by (3-mercaptopropyl) trimethoxysilane, which confirms the synthesis process was successful.Back titration of Fe3O4@ZrO2-PrSO3H in aqueous media
Acidity [H+] of synthesized catalyst (Fe3O4@ZrO2-PrSO3H) was indicated by back titration method. The procedure of this titration include: 0.5 g of catalyst, 0.5 g of NaCl and 10 mL of NaOH (0.1 M) were added to 20 mL distilled water and stirred for 24 h on a magnetic stirrer. After that, three drops phenolphthalein was added to it and colour of mixture was pink. Then it was titrated with solution of HCl (0.1 M) until reached to neutral point. After calculations, pH value of catalyst was obtained 2.25.Surface acidity studies
The acidity strength of an acid in organic solvents can be declared by the Hammett acidity function (H0).38,39 It can be calculated by the following equation:| H0 = pK(I)aq + log([I]s/[IH+]s) |
Here, we used 4-nitroaniline as the basic indicator and CCl4 was chosen as the solvent. The maximal absorbance of the unprotonated form of 4-nitroaniline was observed at 329 nm in CCl4. As can be resulted from Fig. 6, the indicator was partially in the form as [IH+] (the absorbance of the unprotonated form of the indicator in Fe3O4@ZrO2-Pr-SO3H was weak as compared to the sample of the indicator in CCl4). The achieved results of the acidity strength of Fe3O4@ZrO2-Pr-SO3H are listed in Table 1 and absorption spectra of 4-nitroaniline and Fe3O4@ZrO2-Pr-SO3H can be seen in Fig. 6.
 | ||
| Fig. 6 Absorption spectra of 4-nitroaniline (indicator; curve A) and Fe3O4@ZrO2-Pr-SO3H (catalyst; curve B) in CCl4. | ||
| Entry | Catalyst | Amax | [I]s (%) | [IH+]s (%) | H0 |
|---|---|---|---|---|---|
| a Condition for UV-visible spectrum measurement: solvent, CCl4; indicator, 4-nitroaniline (pK(I)aq: 0.99), 1.44 × 10−4 mol per litre; catalyst, Fe3O4@ZrO2-Pr-SO3H, 20 mg, 25 °C. | |||||
| 1 | — | 2.147 | 100 | 0 | — |
| 2 | Fe3O4@ZrO2-Pr-SO3H | 0.933 | 43.45 | 56.55 | 0.876 |
Magnetic properties for Fe3O4@ZrO2-Pr-SO3H
Results of the VSM measurement for bare Fe3O4 and Fe3O4@ZrO2-Pr-SO3H have been reported (Fig. 7). The hysteresis loop for Fe3O4@ZrO2-Pr-SO3H with limited filed from −1000 to 1000 Oe was shown in Fig. 7b. According to this figure, regarding to the first amount of magnetization saturation of Fe3O4 which was 61 emu g−1, due to the coating of surface of Fe3O4 by propyl sulphate, the amount of magnetization saturation of Fe3O4@ZrO2-Pr-SO3H was decreased to 13.96 emu g−1. This clearly has shown that the coating process successfully was carried out.Result and discussion
The trimethylsilylation of hydroxyl alcohols groups simply carried out in the not extreme conditions, solvent-free and room temperature in the presence catalyst Fe3O4@ZrO2-Pr-SO3H. The possible mechanism has been shown in Scheme 3. This mechanism is based-on Brønsted acid and explains interactions between (–SO3H) groups and nitrogen in HMDS that reactive silylating agent.Alcohols or phenols (1 mmol) could be added to a stirring mixture of Fe3O4@ZrO2-Pr-SO3H (10 mg) and HMDS (0.7 mmol) at r.t (Scheme 1). After accomplishment of the reaction, catalyst was separated simply from of reaction mixture by a magnet. The optimized catalyst amount was determined by mixing benzyl alcohol, HDMS and different amounts of the catalysts. The mixtures were stirred at room temperature and solvent free conditions. The results of this optimisation were presented in Table 2. According to results, the reaction can be carried out in the presence catalyst in the less time and the yields are outstanding. This catalyst has higher efficiency than previous reported ones. Also this reaction will be considered as a green reaction due to the solvent free conditions. According to Table 2; the optimized amount of catalyst was 10 mg.
| Entry | Amount of catalyst (mg) | Time (min) | Yield (%) |
|---|---|---|---|
| a Reaction of benzyl alcohol (1 mmol) and HMDS (0.7 mmol, 0.112 g) in the present of different amounts of Fe3O4@ZrO2-Pr-SO3H at room temperature under solvent-free conditions. | |||
| 1 | — | 90 | 85 |
| 2 | 5 | 12 | 99 |
| 3 | 8 | 10 | 99 |
| 4 | 10 | 6 | 99 |
| 5 | 15 | 6 | 99 |
In order to show productivity of Fe3O4@ZrO2-Pr-SO3H, various catalysts such as trichloroisocyanuricacid (TCCA),40 CuSO4·5H2O,41 MgBr2·OEt2,20 iodine,42 H3PW12O40,43 ZrCl4,18 ZrO(OTf)2,44 sulfonic acid@nanoporous silica,22 LaCl3,21 HClO4–SiO2 (ref. 45) and [VIV(TPP)(OTf)2]23 were also used for protection of benzyl alcohol by HMDS. The results were given in Table 3. According to this table, Fe3O4@ZrO2-Pr-SO3H is very superior than the other catalysts because of solvent-free, simple recovery, and environmentally friendly method, low catalyst loading, high efficiency and reusability used.
| Entry | Catalyst | Catalyst load (mg) | Time (min) | Solvent | Yield (%) | Temperature (°C) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Trichloroisocyanuricacid (TCCA) | 24 | 240 | CH2Cl2 | 90 | r.t | 40 |
| 2 | CuSO4·5H2O | 20 | 12 | CH3CN | 98 | r.t | 41 |
| 3 | MgBr2·OEt2 | 50 | 5 | Neat | 98 | r.t | 20 |
| 4 | Iodine | 10 | 2 | CH2Cl2 | 98 | r.t | 42 |
| 5 | H3PW12O40 | 29 | 23 | Neat | 90 | 55–60 | 43 |
| 6 | ZrCl4 | 5 | 1 | CH3CN | 95 | r.t | 18 |
| 7 | ZrO(OTf)2 | 20 | 1 | CH3CN | 92 | r.t | 44 |
| 8 | Sulfonic acid@nanoporous silica | 30 | 55 | CH2Cl2 | 99 | r.t | 22 |
| 9 | LaCl3 | 49 | 180 | CH2Cl2 | 91 | r.t | 21 |
| 10 | HClO4–SiO2 | 15 | 2 | CH3CN | 98 | r.t | 45 |
| 11 | Poly(N-bromobenzene-1,3-disulfonamide) | 20 | 90 | CH2Cl2 | 90 | r.t | 46 |
| 12 | [VIV(TPP)(OTf)2] | 10 | 1 | CH3CN | 100 | r.t | 23 |
| 13 | Fe3O4@ZrO2-Pr-SO3H | 10 | 6 | Neat | 99 | r.t | This work |
Results of these reactions with various alcohols were summarized in Table 4. According to this table, the different alcohols was used in this research such as benzylic, primary, secondary, and phenolic, and got the best result on the yields and time of the reaction. The achieved results showed that electron-donating alcohols required less reaction times in comparison to electron withdrawing alcohols. Also secondary alcohols reacted slowly at room temperature than primary alcohols (Table 4). In addition to, the results displays that aliphatic alcohols need more reaction time compared to aromatic alcohols. The reaction progress was monitored by TLC, GC and FT-IR in order to demonstrate complete protection of hydroxyl. The results showed that all reactions were perfect in the 6–10 min in the solvent-free conditions and less reaction time for all alcohols and also the extraordinary yields at (r.t) were obtained. Also it has high efficiency chemoselective for hydroxyl group but it was not successful to protect thiol and amine groups. Also recovered catalyst by magnet was washed with acetone and distilled water and then dried at 110 °C for 30 min. Afterward, reutilize for more 10 successive runs under same reaction conditions. These results exhibit the highest stability and also the least change in the activity of the catalyst as shown in Fig. 8. One of the reasons for decreasing of the catalytic activities is reduction the acidities of catalyst. To prove this, the acidity was tested for each reaction using back titration. The results indicated the little reduction in the catalyst acidity in each reaction.
| Entry | Substrate | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: alcohol (1 mmol), catalyst (10 mg), HMDS (0.7 mmol, 0.112 g), room temperature (RT).b Yield were determined by GC. | ||||
| 1 | 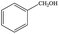 |
 |
6 | 99≥ |
| 2 |  |
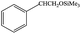 |
6 | 99≥ |
| 3 | 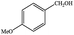 |
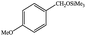 |
6 | 99≥ |
| 4 | 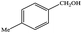 |
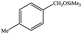 |
6 | 99≥ |
| 5 | 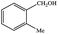 |
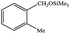 |
6 | 99≥ |
| 6 |  |
 |
8 | 99≥ |
| 7 |  |
 |
8 | 99≥ |
| 8 |  |
 |
9 | 99≥ |
| 9 |  |
 |
9 | 99≥ |
| 10 |  |
 |
7 | 99≥ |
| 11 |  |
 |
6 | 99≥ |
| 12 |  |
 |
6 | 99≥ |
| 13 |  |
 |
10 | 99≥ |
| 14 |  |
 |
6 | 99≥ |
| 15 |  |
 |
6 | 99≥ |
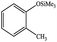
| 16 |  |
 |
7 | 99≥ |
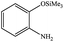
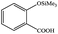
| 17 | 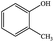 |
 |
6 | 99≥ |
| 18 | 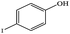 |
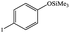 |
7 | 99≥ |
| 19 | 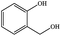 |
 |
6 | 99≥ |
| 20 |  |
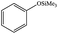 |
6 | 99≥ |
| 21 | 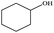 |
 |
7 | 99≥ |
| 22 |  |
 |
8 | 99≥ |
| 23 |  |
 |
8 | 99≥ |
| 24 |  |
 |
8 | 99≥ |
Conclusion
In conclusion, Fe3O4@ZrO2-Pr-SO3H acid has been exhibited to be an efficient and suitable catalyst. Also green procedure for the protection of alcohols and phenols by HMDS in high yield, solvent-free conditions, safe and environmentally benign method. To conclude, simple recovery, simple work up, mild conditions, short reaction time, inexpensive of catalyst and high efficiency make our method to be effective and appropriate for protection alcohols and aliphatic alcohols.Acknowledgements
The authors would like to thank Iran University of Science and Technology, and Iran Nanotechnology Initiative Council for financial support of this work.References
- H. Ghafuri, A. Rashidizadeh, B. Ghorbani and M. Talebi, New J. Chem., 2015, 39, 4821–4829 RSC.
- D. J. Cole-Hamilton, Science, 2003, 299(5613), 1702–1706 CrossRef CAS PubMed.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98(1), 199–218 CrossRef CAS PubMed.
- L. Kljajević, B. Matović, A. Radosavljević-Mihajlović, M. Rosić, S. Bosković and A. Devečerski, J. Alloys Compd., 2011, 509(5), 2203–2215 CrossRef.
- H. Liu, X. Sun, C. Yin and C. Hu, J. Hazard. Mater., 2008, 151(2), 616–622 CrossRef CAS PubMed.
- Z. Zhao, J. Liu, F. Cui, H. Feng and L. Zhang, J. Mater. Chem., 2012, 22, 9052 RSC.
- N. N. Li, T. F. Kang, J. J. Zhang, L. P. Lu and S. Y. Cheng, Anal. Methods, 2015, 7, 5053–5059 RSC.
- T. Watahiki, M. Matsuzaki and T. Oriyama, Green Chem., 2003, 5(1), 82–84 RSC.
- H. Ito, K. Takagi and T. Miyahara, Org. Lett., 2005, 7(14), 3001–3004 CrossRef CAS PubMed.
- T. Suzuki, T. Watahiki and T. Oriyama, Tetrahedron Lett., 2000, 41(46), 8903–8906 CrossRef CAS.
- A. Ghorbani-Choghamarani, M. A. Zolfigol, M. Hajjami and S. Jafari, J. Chin. Chem. Soc., 2008, 55(6), 1208–1213 CrossRef CAS.
- A. Ghorbani-Choghamarani, K. Amani, M. A. Zolfigol, M. Hajjami and R. Ayazi-Nasrabadi, J. Chin. Chem. Soc., 2009, 56(2), 255–260 CrossRef CAS.
- J. Cossy and P. Pale, Tetrahedron Lett., 1987, 28(48), 6039–6040 CrossRef CAS.
- J. Schölmerich, E. Schmidt, C. Schümichen, P. Billmann, H. Schmidt and W. Gerok, Gastroenterology, 1988, 95(5), 1287–1293 CrossRef.
- C. A. Bruynes and T. Jurriens, J. Org. Chem., 1982, 47(20), 3966–3969 CrossRef CAS.
- M. Moghadam, S. Tangestaninejad, V. Mirkhani, I. Mohammadpoor Baltork and S. Gharaati, Appl. Organomet. Chem., 2009, 23(11), 446–454 CrossRef CAS.
- K. Kulangiappar, M. Anbukulandainathan and T. Raju, Synth. Commun., 2014, 1(44), 2494–2502 CrossRef.
- F. Shirini and E. Mollarazi, Catal. Commun., 2007, 8(9), 1393–1396 CrossRef CAS.
- N. Azizi and M. R. Saidi, Organometallics, 2004, 23(6), 1457–1458 CrossRef CAS.
- M. M. Mojtahedi, H. Abbasi and M. S. Abaee, J. Mol. Catal. A: Chem., 2006, 250(1), 6–8 CrossRef CAS.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98(1), 199–218 CrossRef CAS PubMed.
- D. Zareyee and B. Karimi, Tetrahedron Lett., 2007, 48(7), 1277–1280 CrossRef CAS.
- M. Moghadam, I. Mohammadpoor-Baltork, S. Tangestaninejad, V. Mirkhani, A. R. Khosropour and S. A. Taghavi, Appl. Organomet. Chem., 2011, 25(9), 687–694 CAS.
- S. Atghia and S. S. Beigbaghlou, J. Organomet. Chem., 2013, 745, 42–49 CrossRef.
- F. Hoffmann, M. Cornelius, J. Morell and M. Fröba, Angew. Chem., Int. Ed., 2006, 45(20), 3216–3251 CrossRef CAS PubMed.
- P. Gholamzadeh, G. M. Ziarani, N. Lashgari, A. Badiei and P. Asadiatouei, J. Mol. Catal. A: Chem., 2014, 391, 208–222 CrossRef CAS.
- F. Nikbakht, E. Ghonchepour, H. Ziyadi and A. Heydari, RSC Adv., 2014, 4(65), 34428–34434 RSC.
- B. Das, K. Venkateswarlu, M. Krishnaiah and H. Holla, Tetrahedron Lett., 2006, 47(49), 8693–8697 CrossRef CAS.
- F. Sannino, D. Pirozzi, A. Aronne, E. Fanelli, R. Spaccini, A. Yousuf and P. Pernice, Environ. Sci. Technol., 2010, 44(24), 9476–9481 CrossRef CAS PubMed.
- A. Sarkar, S. K. Biswas and P. Pramanik, J. Mater. Chem., 2010, 20(21), 4417–4424 RSC.
- T. Wu, J. Wan and X. Ma, Chin. J. Catal., 2015, 36, 425–431 CrossRef CAS.
- H. Jiang, P. Chen, W. Zhang, S. Luo, X. Luo, C. (Peter) Au and M. Li, Appl. Surf. Sci., 2014, 317, 1080–1089 CrossRef CAS.
- H. Jiang, P. Chen, S. Luo, X. Tu, Q. Cao and M. Shu, Appl. Surf. Sci., 2013, 284, 942–949 CrossRef CAS.
- S. Zhang, Y. Zhang, J. Liu, Q. Xu, H. Xiao, X. Wang and H. Xu, Chem. Eng. J., 2013, 226, 30–38 CrossRef CAS.
- Q. Qu, Q. Gu, Z. Gu, Y. Shen, C. Wang and X. Hu, Colloids Surf., A, 2012, 415, 41–46 CrossRef CAS.
- K. Miyatake, H. Iyotani, K. Yamamoto and E. Tsuchida, Macromolecules, 1996, 29(21), 6969–6971 CrossRef CAS.
- R. Langner and G. Zundel, J. Phys. Chem., 1995, 99(32), 12214–12219 CrossRef CAS.
- A. Amoozadeh, S. Golian and S. Rahmani, RSC Adv., 2015, 5, 45974–45982 RSC.
- H. Xing, T. Wang, Z. Zhou and Y. Dai, J. Mol. Catal. A: Chem., 2007, 264, 53–59 CrossRef CAS.
- A. Khazaei, et al., Catal. Commun., 2007, 8(3), 543–547 CrossRef CAS.
- M. Alvaro, A. Corma, D. Das, V. Fornés and H. García, J. Catal., 2005, 231(1), 48–55 CrossRef CAS.
- B. Karimi and B. Golshani, J. Org. Chem., 2000, 65(21), 7228–7230 CrossRef CAS PubMed.
- H. Firouzabadi, N. Iranpoor, K. Amani and F. Nowrouzi, J. Chem. Soc., Perkin Trans. 1, 2002, 23, 2601–2604 RSC.
- M. Moghadam, S. Tangestaninejad, V. Mirkhani, I. Mohammadpoor-Baltork, S. Chahardahcheric and Z. Tavakoli, J. Organomet. Chem., 2008, 693(11), 2041–2046 CrossRef CAS.
- H. R. Shaterian, F. Shahrekipoor and M. Ghashang, J. Mol. Catal. A: Chem., 2007, 272(1), 142–151 CrossRef CAS.
- R. Ghorbani-Vaghei, M. A. Zolfigol, M. Chegeny and H. Veisi, Tetrahedron Lett., 2006, 47(26), 4505–4508 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |









