Synthesis of phosphorus amidite ligand and investigation of its flexibility impact on rhodium-catalyzed hydroformylation of 1-octene†
Weibiao Hana,
Song Qina,
Xiao Shua,
Qianhui Wua,
Bin Xub,
Ruixiang Lia,
Xueli Zheng*a and
Hua Chen*a
aKey Lab of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, China. E-mail: scuhchen@163.com; 14359385@qq.com
bKey Laboratory of Green Chemistry of Sichuan Institutes of Higher Education, Zigong 643000, China
First published on 25th May 2016
Abstract
A new flexible 2,2′-bis((dipyrrolylphosphinooxy)methyl)-1,1′-(±)-biphenyl ligand (L1) was synthesized, characterized and employed in the hydroformylation of 1-octene. In order to investigate the influence of ligand flexibility on the catalytic performance, its analogue 2,2′-bis (dipyrrolylphosphinooxy)-1,1′-(±)-biphenyl (L3) was also applied in the hydroformylation of 1-octene. With the presence of L1, the aldehyde selectivity was approximately 10% higher than that with the relevant less flexible ligand L3. Theoretical calculation indicated that the olefin-insertion into the Rh–H bond of intermediate III and the CO-insertion into Rh–alkyl bond of intermediate V were favorable in terms of thermodynamics, which were vital to the generation of aldehyde. Meanwhile, the ligand flexibility was indeed improved by adding a methylene between benzene and oxygen atom that connected with P, as the calculation showed the variation of the bite angle ∠P–Rh–P of the intermediates I–VI was 8.7° in L1-system, as for L3-system, the variation was 14.0°. This structural feature might make the olefin-insertion and the CO-insertion occur more easily in the L1-system and resulted in higher aldehyde selectivity.
Introduction
Hydroformylation of olefins remains one of the most important chemical reactions. It has been widely explored under the catalysis of rhodium catalysts for the production of aldehydes.1 Mostly, the aldehydes could be used to make other chemical products such as plasticisers, pharmaceuticals and lubricants.2 Recently, extensive research has been devoted to ligand modification, as the stereoelectronic properties of ligands have a remarkable influence on the catalytic performance in the rhodium-catalyzed hydroformylation.3–6 Ligands possessing strong π-acceptor were found to be effective in terms of regioselectivity and activity.7,8 For example, van Leeuwen has developed a novel bidentate ligand, 1,1′-biphenyl-2,2′-diyl-bis(dipyrrolylphosphoramidite), which led to high selectivity and activity in Rh-catalyzed hydroformylation of 1-octene due to bearing the electron-withdrawing pyrrolyl substituents.9 Zhang and co-workers reported that its triphosphorus10–12 and tetraphosphorus13 analogue capable of forming multiple chelating modes with Rh, could provide better regioselectivity in isomerization-hydroformylation of internal olefins with the presence of Rh(acac)(CO)2 (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i values could reach up to 47.0 and 51.7 for 2-octene with triphosphorus and tetraphosphorus ligands, respectively).
i values could reach up to 47.0 and 51.7 for 2-octene with triphosphorus and tetraphosphorus ligands, respectively).
van Leeuwen found that the natural bite angle ∠P–Rh–P in L2Rh(CO)H(alkene) (L = ligand) had close relationship with activity and selectivity.14 A better regioselectivity towards the linear aldehyde could be obtained when increasing the natural bite angle to a certain degree.6,15 Although the complex (L)2Rh(CO)H(alkene) has never been captured directly, many research revealed that wide-bite-angle diphosphite ligands, such as BISBI-based bidentate ligands, the xanthenes family of ligands, diary bicycle [2.2.n] compounds16 and modified biphenyl-based bisphosphites ligands,17 favored the formation of linear alkyl rhodium species6 and thus the linear aldehyde was preferred. Dieter Vogt et al.18 studied a series of sterically demanding xanthenes-based diphosphites ligands and found that more flexible ligands exhibited high activities as well as good selectivities to linear aldehyde in the Rh-catalyzed hydroformylation of 1-olefin. The crystal structure revealed that less flexible ligand aroused weak interaction between the O atom of xanthene and Rh center, thus the hydroformylation result was inferior to that with the more flexible ligand.
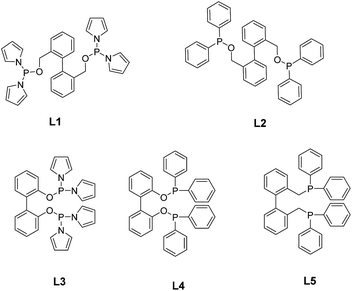
Inspired by the above reports, the electron-withdrawing pyrrolyl substituent would improve the π-acceptor ability of the phosphorus ligand. Herein, we attempt to synthesize pyrrolyl-substituted diphosphine ligands with biphenyl as the backbone, and introduce –CH2– between benzene ring and oxygen atom to adjust the flexibility (L1, Fig. 1), we envision that this ligand could serve as an effective ligand for the hydroformylation of 1-octene. DFT calculation revealed that the introduction of –CH2– modified the flexibility of the ligand, influenced the bite angle ∠P–Rh–P and favored the insertion of olefin and CO, and consequently, increased the selectivity to aldehyde.
Experimental
Synthesis of backbones and ligands
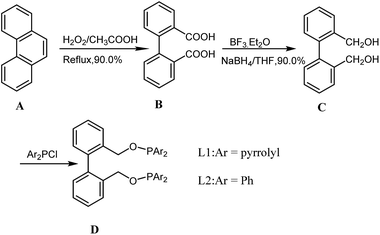
The synthesis of ligands L1 and L2 were realized as shown in Scheme 1. Starting from the oxidation of phenanthrene (A) by H2O2,21 reduction of 2,2′-biphenyldimenthanoic acid (B) by sodium borohydride 22, and the reaction of diol (C) with chlorodiarylphosphine afforded the desired phosphane ligands L1 and L2. The two compounds were unambiguous proven by means of 1H, 13C and 31P NMR, as well as mass spectrometry.Results and discussion
Hydroformylation of 1-octene
Hydroformylation of 1-octene with the new ligands L1 and L2 was then investigated, respectively. The catalytic condition was explored by using L1 as the benchmark ligand. The rhodium catalyst was prepared in situ by mixing L1 with Rh(acac)(CO)2 (acac = acetylacetonate) in toluene. The reaction proceeded in toluene and the products were analyzed by gas chromatography. The Rh(acac)(CO)2 concentration was 1.59 mM. The effects of ligand/metal ratio on hydroformylation of 1-octene were studied and the results were shown in Table 1 (entries 1–6).| Entry | P/Rh | T (°C) | P (MPa) | Conv.b (%) | Aldehydec (%) | Oct.d (%) | Iso.e (%) | n/if |
|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: S/C ratio of entry 1–6 is 2000 and others are 1000, [Rh] = 1.60 mM, toluene 3 ml, 1 h.b The conversion of 1-octene.c Selectivity for aldehyde in all product.d Selectivity for octane in all product.e Selectivity for 2-octene in all product.f Molar ratio of linear to branched aldehyde. The products were analyzed by GC. | ||||||||
| 1 | 0 | 80 | 1.5 | 78.0 | 16.4 | 61.0 | 22.6 | 2.7 |
| 2 | 5 | 80 | 1.5 | 97.8 | 64.9 | 17.6 | 17.5 | 2.8 |
| 3 | 10 | 80 | 1.5 | 97.2 | 85.2 | 1.8 | 13.0 | 9.0 |
| 4 | 15 | 80 | 1.5 | 87.5 | 89.3 | 0.7 | 10.0 | 10.6 |
| 5 | 20 | 80 | 1.5 | 71.3 | 90.0 | 1.0 | 9.0 | 10.8 |
| 6 | 25 | 80 | 1.5 | 63.3 | 88.5 | 1.0 | 10.5 | 10.7 |
| 7 | 10 | 60 | 1.5 | 26.9 | 94.1 | 3.5 | 2.4 | 10.5 |
| 8 | 10 | 80 | 1.5 | 96.5 | 91.9 | 3.3 | 4.8 | 10.8 |
| 9 | 10 | 100 | 1.5 | 98.2 | 86.4 | 8.1 | 5.5 | 8.3 |
| 10 | 10 | 120 | 1.5 | 98.4 | 82.0 | 8.2 | 9.8 | 5.5 |
| 11 | 5 | 70 | 0.5 | 97.2 | 80.4 | 3.5 | 16.1 | 9.7 |
| 12 | 5 | 70 | 1.0 | 98.1 | 89.5 | 1.5 | 9.0 | 9.4 |
| 13 | 5 | 70 | 2.0 | 97.6 | 94.1 | 0.5 | 5.4 | 9.2 |
| 14 | 5 | 70 | 3.0 | 97.8 | 95.2 | 0.4 | 4.4 | 8.4 |
The linear/branched (n/i) ratio increased from 2.7 to 10.6 and the aldehyde selectivity increased from 16.4 to 89.3 when the ligand/metal ratio was increased from 0 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
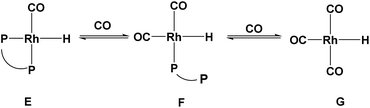
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, a decrease in the isomerization (22.6 to 10.0) and hydrogenation (61.0 to 0.7) was detected (Table 1, entries 1–4). Further increasing the ligand/metal ratio did not improve the regioselectivity, but resulted in a little drop of conversion (Table 1, entries 4–6). According to ligand dissociation in Rh-catalyzed hydroformylation,10 increasing the local phosphine concentration around the rhodium atom could enhance the coordination between P and Rh to form complex E (Scheme 2), which would benefit the reaction activity and the selectivity to aldehyde.19 However, more excess ligand would block the coordination site and the coordination of 1-octene with rhodium active species would become difficult. Therefore, further increasing the ligand/metal ratio would cause its activity to drop. Decreasing the ligand concentration might form multiple carbonyls complex F and G (Scheme 2), which was not conductive to the formation of aldehyde.
1. However, a decrease in the isomerization (22.6 to 10.0) and hydrogenation (61.0 to 0.7) was detected (Table 1, entries 1–4). Further increasing the ligand/metal ratio did not improve the regioselectivity, but resulted in a little drop of conversion (Table 1, entries 4–6). According to ligand dissociation in Rh-catalyzed hydroformylation,10 increasing the local phosphine concentration around the rhodium atom could enhance the coordination between P and Rh to form complex E (Scheme 2), which would benefit the reaction activity and the selectivity to aldehyde.19 However, more excess ligand would block the coordination site and the coordination of 1-octene with rhodium active species would become difficult. Therefore, further increasing the ligand/metal ratio would cause its activity to drop. Decreasing the ligand concentration might form multiple carbonyls complex F and G (Scheme 2), which was not conductive to the formation of aldehyde.
The effects of temperature and CO/H2 pressure on the Rh-catalyzed hydroformylation reaction were also tested. To our delight, the aldehyde selectivity was comparably higher at lower temperature. An obvious decrease in both n/i and aldehyde selectivity were observed when the reaction temperature was increased from 60 to 120 °C (Table 1, entries 7–10). It should be noted that the aldehyde selectivity was 91.9% and the n/i ratio was 10.8 when the hydroformylation was conducted at 80 °C (Table 1, entry 8), and both the olefin isomerization and hydrogenation products (octane) increased. It is well known that hydroformylation at higher temperature might lead to more olefin isomerization and hydrogenation. A high regioselectivity (n/i ratio 9.7) was obtained under a CO/H2 pressure of 0.5 MPa, however, a significant amount of isomerization was observed under this pressure, indicating that low CO/H2 pressure could in turn facilitate the olefin isomerisation. Increasing the CO/H2 pressure from 0.5 to 3 MPa (Table 1, entries 11–14) did not obviously improve the regioselectivity but increase the aldehyde selectivity to 95.2%. A similar tendency was observed when L1 was replaced by L2 (Table 3) (see ESI†). In order to explore the impact of flexibility and the electronic properties on the catalytic performance, ligands L2–L5 were employed in the hydroformylation of 1-octene with the presence of Rh(acac)(CO)2, and the results were summarized in Table 2. All the ligands demonstrated good reaction activities and moderate regioselectivities. However, the catalytic performance of Rh/L1 was dramatically superior to Rh/L2, that is, under optimized reaction condition, the aldehyde selectivity and the n/i ratio could reach up to 91.9% and 10.8, respectively, probably as a result of the easy carbon monoxide dissociation and stronger alkene coordination20–22 owing to bearing the electron withdrawing pyrrolyl. In addition, L3 also possessed of pyrrolyl group but afforded higher regioselectivity along with lower aldehyde selectivity compared with L1 (Table 2), that is, the n/i ratio was 93.9 when L3 was used as the ligand, correspondingly, and 10.8 was afforded by L1. But it should be noted that when L1 was used, the aldehyde selectivity is about 10.0% higher than that with L3 (Table 2,† entry 1 vs. entry 3). The difference between L1 and L3 is that –CH2– was inserted between the benzene ring and the oxygen atom in L1, for the aim of increasing the flexibility of the ligand and improving the regioselectivity and activity, although the addition of –CH2– did not benefit the regioselectivity for 1-octene hydroformylation. Similarly, L2 also afforded lower n/i and higher aldehyde selectivity compared with L4.
| Entry | Ligand | Con.b (%) | Aldehyde yieldc (%) | Oct.d (%) | Iso.e (%) | n/if |
|---|---|---|---|---|---|---|
| a Reaction conditions: S/C = 1000, T = 80 °C, L/Rh = 10, P = 1.5 MPa, n(CO)/n(H2) = 1, [Rh] = 1.6 mM, toluene 3 ml, t = 1 h.b The conversion of 1-octene.c Selectivity for aldehyde in all product.d Selectivity for octane in all product.e Selectivity for 2-octene in all product.f Molar ratio of linear to branched aldehyde. The products were analyzed by GC. | ||||||
| 1 | L1 | 96.5 | 91.9 | 3.3 | 4.8 | 10.8 |
| 2 | L2 | 89.7 | 70.0 | 21.7 | 8.3 | 2.6 |
| 3 | L3 | 97.4 | 80.9 | 11.5 | 7.6 | 93.9 |
| 4 | L4 | 78.0 | 51.9 | 27.6 | 20.5 | 7.4 |
| 5 | L5 | 79.8 | 90.0 | 5.4 | 4.6 | 61.0 |
Mechanism by DFT calculation and discussion
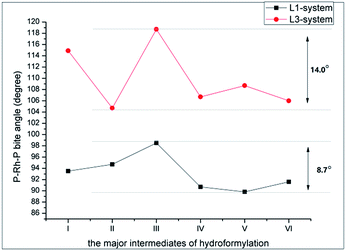
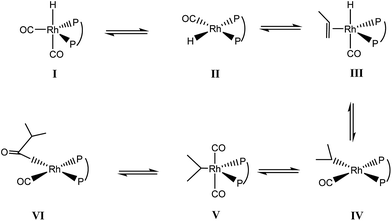
To obtain insight information about the influence of ligands with different flexibility on the present Rh-catalyzed hydroformylation process, the DFT calculation with the emphasis on the geometric properties of the various active catalytic species of L–Rh(CO)2H (L = L1 or L3) was conducted at the M06/6-31G**, LanL2DZ level. M0623 method combined with the Lanl2DZ basis set24 for Ph and 6-31G** basis set25 for the rest atoms was employed in the gas-phase simulation. The optimization on the similar compounds BISBI–Rh(PPh3)(CO)H whose crystal structure parameters are available in the reported literature,26 was performed to test the reliability of this method. The calculation shows that the derivation in the length of the key bonds is less than 0.05 Å, indicating that M06 method is suitable for the present system. To reduce computational cost, the octene was replaced by propene as the substrate for the present calculation.DFT calculation predicted that the catalytic process starts from the formation of active species of L–Rh(CO)2H. The bite angle of P–Rh–P is predicted to be 93.5° for L1–Rh(CO)2H, while that of L3–Rh(CO)2H is supposed to be 114.9°. L–Rh(CO)2H complex was reported to be a mixture of the equatorial-apical (ea) and diequatorial (ee) isomers.20,26 The equilibrium composition of the isomers depended on the bite angle of P–Rh–P. In L3–Rh(CO)2H, ∠P–Rh–P 114.9° might make ee isomer predominate which causes linear aldehyde readily to generate.15 Nevertheless, ∠P–Rh–P 93.5° might make ea isomer predominate in L1–Rh(CO)2H and result in lower regioselectivity towards linear aldehyde (the n/i ratio for Rh/L3 is 93.9 and Rh/L1 is 10.8, Table 3†). In general, the temperature would also influence the equilibrium composition and ee isomer is favourable at high temperature (high n/i ratio). Nevertheless, an opposite trend to the Rh/L1 catalyst system was observed at a lower temperature (Table 1, entry 7–10). The electron-withdrawing ligand L1 might exert a strong chelate effect on Rh atom and could stabilize the catalytic active species (the ee isomers),27,28 which expresses a good linear aldehyde selectivity at a lower temperature. On the other hand, higher temperatures might cause dissociation of the ligand in Rh-catalyzed hydroformylation, which is unfavourable the regioselectivity.
The evolution of the calculated bite angle of the major intermediates from I to V is depicted in Fig. 2. As shown in this figure, the geometric evolution from I to V involves in the penta-coordinated and tetra-coordinated Rh species, and the corresponding bite angle of P–Rh–P in compounds (I–V) varies during the entire reaction. For L1–Rh compounds, the bite angle of P–Rh–P relatively smoothly changes less than 10°. In contrast, this bite angle of L3–Rh system drastically frustrates in the range of 14.0° as compared with L1–Rh system. L1–Rh compounds seem more flexible, which might facilitate the dissociation and coordination processes, and therefore improve the reaction rate of the hydroformylation. It should be noted that the step from III to IV represents the insertion of olefin into Rh–H bond (shown in Scheme 3), and transform of V to VI corresponds to the insertion step of CO. The above two steps are very vital for the formation of the aldehyde. M06 calculation predicts that the ΔH of the olefin-migration step to be −43.4 kJ mol−1 for Rh/L1 system and −13.2 kJ mol−1 for Rh/L3 system, and the ΔH of the CO-insertion step to be −27.3 kJ mol−1 for Rh/L1 system and 43.8 kJ mol−1 for Rh/L3 system, respectively. As a result, the insertion of olefin and CO step could be easier to occur in the Rh/L1 system than Rh/L3 system in thermodynamics. The above two aspects might be the reason for higher aldehyde selectivity which was afforded by Rh/L1 compared to Rh/L3 system.
In addition, the regioselectivity was lower at higher CO/H2 pressure (Table 1, entries 11–14). It could be explained by the reason that the phosphorus atoms in the complex IV could be replaced by CO, which was against the generation of linear aldehyde with the concentration of CO increasing.8,21
Conclusion
On the basis of the unique electronic properties of N-pyrrolylphosphorus ligand, –CH2– moiety was introduced to modify the flexibility of the ligand. L1 was synthesized and applied to Rh-catalyzed hydroformylation of 1-octene. Under optimized reaction condition (80 °C, 1.5 MPa, L/Rh = 10, S/C = 1000, 1 h), the aldehyde selectivity and the n/i ratio could reach up to 91.9% and 10.8, respectively. The results revealed that the increase of flexibility was beneficial to the selectivity towards aldehyde. And calculation showed the olefin-insertion into the Rh–H bond of intermediate III and CO-insertion into Rh–alkyl bond of intermediate V could be facilitated by the more flexible ligand L1 and thus higher aldehyde selectivity was afforded by Rh/L1 compared to Rh/L3 system.Acknowledgements
The authors thank the financial supports from the National Natural Science Foundation of China (201202108), from the Opening Project of Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education (LZJ1402) and from the Sichuan university outstanding scholars research fund (2015SCU04A05).Notes and references
- B. Cornils, W. A. Herrmann and M. Rasch, Angew. Chem., Int. Ed., 1994, 33, 2144 CrossRef.
- M. Sparta, K. J. Børve and V. R. Jensen, J. Am. Chem. Soc., 2007, 129, 8487 CrossRef CAS PubMed.
- P. C. J. Kamer, P. W. N. M. van Leeuwen and J. N. H. Reek, Acc. Chem. Res., 2001, 34, 895 CrossRef CAS PubMed.
- G. Abkai, S. Schmidt, T. Rosendahl, F. Rominger and P. Hofmann, Organometallics, 2014, 33, 3212 CrossRef CAS.
- J. Mormul, M. Mulzer, T. Rosendahl, F. Rominger, M. Limbach and P. Hofmann, Organometallics, 2015, 34, 4102 CrossRef CAS.
- L. A. van der Veen, P. H. Keeven, G. C. Schoemaker, J. N. H. Reek, P. C. J. Kamer, P. W. N. M. van Leeuwen, M. Lutz and A. L. Spek, Organometallics, 2000, 19, 872 CrossRef CAS.
- R. Franke, D. Selent and A. Börner, Chem. Rev., 2012, 112, 5675 CrossRef CAS PubMed.
- R. Jackstell, H. Klein, M. Beller, K.-D. Wiese and D. Röttger, Eur. J. Org. Chem., 2001, 2001, 3871 CrossRef.
- S. C. van der Slot, J. Duran, J. Luten, P. C. J. Kamer and P. W. N. M. van Leeuwen, Organometallics, 2002, 21, 3873 CrossRef CAS.
- S. Yu, X. Zhang, Y. Yan, C. Cai, L. Dai and X. Zhang, Chem. –Eur. J., 2010, 16, 4938 CrossRef CAS PubMed.
- C. Chen, Y. Qiao, H. Geng and X. Zhang, Org. Lett., 2013, 15, 1048 CrossRef CAS PubMed.
- C. Chen, P. Li, Z. Hu, H. Wang, H. Zhu, X. Hu, Y. Wang, H. Lv and X. Zhang, Org. Chem. Front., 2014, 1, 947 RSC.
- Y. Yan, X. Zhang and X. Zhang, J. Am. Chem. Soc., 2006, 128, 16058 CrossRef CAS PubMed.
- C. P. Casey and G. T. Whiteker, Isr. J. Chem., 1990, 30, 299 CrossRef CAS.
- E. Zuidema, P. E. Goudriaan, B. H. G. Swennenhuis, P. C. J. Kamer, P. W. N. M. van Leeuwen, M. Lutz and A. L. Spek, Organometallics, 2010, 29, 1210 CrossRef CAS.
- (a) H. Bohnen, J. Herwig, A. Joerg, D. Hoff, S. Surm, P. W. N. W. van Leeuwen, R. Bronger and O. Stelzer, Celanese Chemicals Europe GmbH, DE 10225282, 2003; (b) W. Ahlers, R. Paciello, D. Vogt and P. Hofmann, BASF Aktiengesellschaft, WO 2002083695, 2002; (c) P. Rainer, W. Ahlers, T. Mackewitz, R. Paciello and M. Volland, BASF Aktiengesellschaft, WO 2005039762, 2005; (d) P. W. N. M. van Leeuwen, E. B. Walczuk-Gusciora, N. E. Grimmer and P. C. J. Kamer, Sasol Technology Proprietary Limited, WO 2005049537, 2005.
- C. Andrea, F. Robert, F. Dirk, H. Dieter and D. K. Marie, Hannebauer bernd (Evonik Industries AG), US2015224488, 2015.
- J. I. van der Vlugt, R. Sablong, P. C. Magusin, A. M. Mills, A. L. Spek and D. Vogt, Organometallics, 2004, 23, 3177 CrossRef CAS.
- L. Zhang, C. Li, X. L. Zheng, H. Y. Fu, H. Chen and R. X. Li, Catal. Lett., 2014, 144, 1074 CrossRef CAS.
- L. A. van der Veen, M. D. K. Boele, F. R. Bregman, P. C. J. Kamer, P. W. N. M. van Leeuwen, K. Goubitz, J. Fraanje, H. Schenk and C. Bo, J. Am. Chem. Soc., 1998, 120, 11616 CrossRef CAS.
- W. R. Moser, C. J. Papile, D. A. Brannon, R. A. Duwell and S. J. Weininger, J. Mol. Catal., 1987, 41, 271 CrossRef CAS.
- T. Jongsma, G. Challa and P. Van Leeuwen, J. Organomet. Chem., 1991, 421, 121 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 CrossRef CAS.
- M. Dupuis, J. Rys and H.-F. King, J. Chem. Phys., 1976, 65, 111 CrossRef CAS.
- G. A. Petersson, A. Bennett, T. G. Tensfeldt, M. A. Al-Laham, W. A. Shirley and J. Mantzaris, J. Chem. Phys., 1988, 89, 2193 CrossRef CAS.
- C. P. Casey and L. M. Petrovich, J. Am. Chem. Soc., 1995, 117, 6007 CrossRef CAS.
- T. Matsubara, N. Koga, Y. Ding, D. G. Musaev and K. Morokuma, Organometallics, 1997, 16, 1065 CrossRef CAS.
- R. Schmid, W. A. Herrmann and G. Frenking, Organometallics, 1997, 16, 701 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C and 31P NMR spectra; the calculated bite angle of the major intermediates; Table 3: optimization of reaction conditions for the hydroformylation of 1-octenewith Rh/L2. See DOI: 10.1039/c6ra09890h |
| This journal is © The Royal Society of Chemistry 2016 |