Recent applications of barbituric acid in multicomponent reactions
Ghodsi Mohammadi Ziarani
*a,
Faezeh Aleali
a and
Negar Lashgari
b
aDepartment of Chemistry, Alzahra University, Tehran, Iran. E-mail: gmziarani@hotmail.com; gmohammadi@alzahra.ac.ir; Fax: +98 21 88041344; Tel: +98 21 88041344
bSchool of Chemistry, College of Science, University of Tehran, Tehran, Iran
First published on 18th May 2016
Abstract
Barbituric acid has been utilized in the design and synthesis of diverse types of heterocyclic and carbocyclic compounds and considered as an important building block in organic synthesis. There is a wide range of multicomponent reactions that include barbituric acid as starting material. This article aims to review the chemistry of barbituric acids employed in the design and synthesis of different types of compounds during the period from 2011 to 2015.
1. Introduction
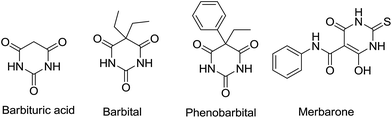
Barbituric acid was first discovered by a German chemist, Adolf von Baeyer in 1864.1 It was synthesized through a condensation reaction of urea with malonic acid. The derivatives of barbituric acid, commonly known as barbiturates, have a special place in pharmaceutical chemistry because of their biological activities such as hypnotic,2,3 sedative,4 anticonvulsant,5,6 antimicrobial,7 anaesthetic,8 anticancer and antitumor properties.9,10 Substances of the barbituric acid group have been used therapeutically for many years. Barbital was the first barbiturate to be developed and is considered a prototype. It is a sedative and hypnotic and was used as a sleeping aid and anxiolytic, although now largely replaced by nonbarbiturate agents.11 Phenobarbital is another medication recommended by the World Health Organization for the treatment of certain types of epilepsy in developing countries (Fig. 1).12 Merbarone was a derivative of barbituric acid and represented a unique class of antineoplastic agents which showed curative activity against L1210 leukemia and also possessed important activity against some other murine tumors (Fig. 1).13Furthermore, barbituric acid and its derivatives have been applied as a new anchor unit for dye-sensitized solar cells.14 Due to the chemical structure of barbituric acid and its capability to make keto–enol form, it has a unique potential to be used as a valuable building block in various organic reactions. The chemistry of barbituric acid was reviewed by Bojarski et al. in 1985.15 Recently a review article on the utility of barbituric acid in multicomponent reactions has been published by Shaker and Ishak in 2011.16 The synthesis, tautomerism, acid–base properties of barbituric acids and their use for the construction of organometallic and coordination compounds, as well as coordination polymers and supramolecular assemblies has been also reviewed.17
Multicomponent reactions are generally defined as reactions where more than two starting materials react to form a product, incorporating essentially all of the atoms of the educts. Such reactions provide a number of valuable conceptual and synthetic advantages over stepwise sequential approaches towards complex and valuable molecules. They are atom economic, efficient, and extremely convergent. Such strategies reduce the number of steps in schemes, thus avoiding the complicated purification procedures and allowing saving of both solvents and reagents.18,19 Owing to the structural importance of barbituric acids in organic synthesis, and since many publications have included barbituric acids in the synthesis of various novel organic compounds over the past five years, this review aims to show representative examples of these reactions reported from the year 2011 to 2015.
2. Synthesis of five-membered heterocycles
2.1. O-Heterocyclic compounds
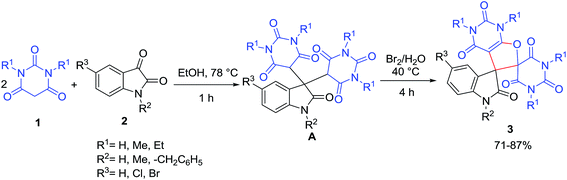
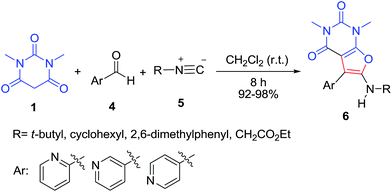
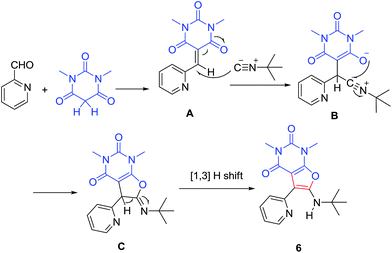
One-pot transformation of barbituric acids 1 and isatins 2 by the direct action of the only bromine in ethanol resulted in the formation of substituted 2′′H-dispiro[indole-3,5′-furo[2,3-d]pyrimidine] system 3 in 71–87% yields via cascade process (Scheme 1).20 The mechanism involves the formation of dibarbiturates A from isatins 2 and barbituric acids 1 as tandem Knoevenagel–Michael reaction. Following cyclization of adduct A by aqueous bromine at 40 °C results in the final product 3.A series of new furo[2,3-d]pyrimidine derivatives 6 were obtained by three-component condensation reaction of isocyanides 5, aldehydes 4 and 1,3-dimethylbarbituric acid 1 at room temperature without any prior activation or modifications (Scheme 2).21 The first step may involve a Knoevenagel condensation between the aldehyde 4 and 1,3-dimethylbarbituric acid 1 for the formation of the stable intermediate enone A, which undergoes nucleophilic attack by isocyanide to generate adduct B. This adduct undergoes intra nucleophilic addition by oxanion for cyclization process of five-member ring to afford compound C which then can undergo tautomerization to output the final compound 6 (Scheme 3).
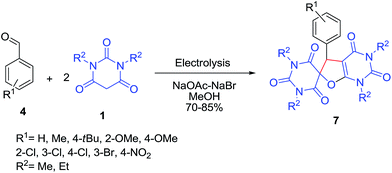
Electrocatalytic assembling of aldehydes 4 and N,N′-dialkylbarbituric acids 1 led to selective formation of substituted spirofuropyrimidines 7 as a result of the complex cascade process (Scheme 4).22 The electrocatalytic process smoothly proceeded with aromatic aldehydes bearing both electron-donating and electron withdrawing groups, and allowed for the selective and efficient one-step formation of spirobarbiturates containing furo[2,3-d]pyrimidine scaffolds 7. Teimouri and co-workers have used urotropine–bromine (UB) complex as catalyst in this reaction to afford the products.23 Pesyan and co-workers reported the reaction of barbituric acid derivatives with cyanogen bromide and aldehydes in the presence of triethylamine24 and L-(+)-tartaric acid25 to afford a new route for the synthesis of spirofuropyrimidines.
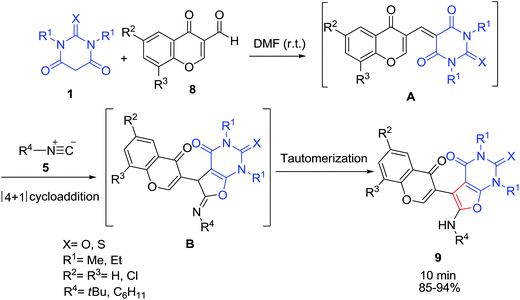
The one-pot three-component reaction of 1,3-disubstituted barbituric acid derivatives 1, 3-formylchromones 8 and alkyl isocyanides 5 proceed with a smooth reaction at room temperature to give the corresponding chromone-containing furopyrimidines derivatives 9 in high yields (Scheme 5).26 The synthesis of these compounds can be rationalised by initial formation of a conjugated electron-deficient heterodiene A by a Knoevenagel condensation of the 1,3-disubstitiuted barbituric acid 1 and 3-formylchromone derivatives 8 followed by a [4 + 1] cycloaddition reaction with isocyanide 5 to afford an iminolactone intermediate B, which then isomerises to yield the final products 9.
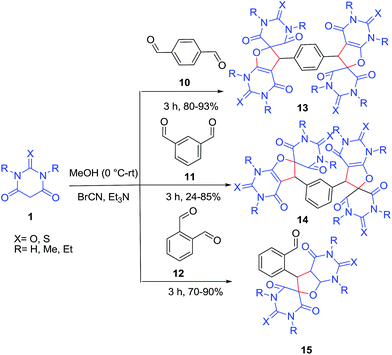
Reaction of barbituric acids 1 with cyanogen bromide and aromatic dialdehydes 10–12 in the presence of triethylamine leads to the selective and efficient formation of a novel class of bi-functionalized stable heterocyclic compounds 13–14 which are dimeric forms of barbiturate linked by a phenyl ring (Scheme 6).27 In contrast, the reaction of phthalaldehyde 12 with BrCN and (thio)barbituric acids resulted in only mono-functionalized spiro[furo[2,3-d]pyrimidine barbiturate] 15.
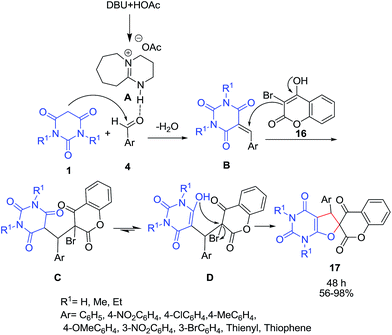
Synthesis of new spiro[chroman-3,6′-furo[2,3-d]pyrimidine]-tetraones 17 by an organocatalyzed three-component condensation reaction of aldehydes 4, barbituric acids 1 and 3-bromo-4-hydroxycoumarin 16 in refluxing acetic acid in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) was reported by Ahadi et al. (Scheme 7).28 In the proposed mechanism for this reaction, first, salt A is formed in situ by acid–base reaction of DBU and HOAc. Then hydrogen bonding is formed between A and the aldehyde 4. This activated aldehyde reacts with barbituric acid 1 by Knoevenagel condensation. Then the Michael addition of 3-bromo-4-hydroxycoumarin 16 to intermediate B followed by cyclization produces the corresponding product 17.
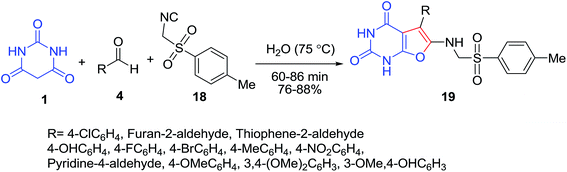
Furo(2,3-d)pyrimidine-2,4(1H,3H)-dione derivatives 19 have been synthesized by three-component condensation of barbituric acid 1, aromatic aldehyde 4, and aryl isocyanide 18 under uncatalyzed conditions in water (Scheme 8).29 The synthesized compounds have been tested for their antifungal and antibacterial activities and most of the compounds exhibited good results.
2.2. N-Heterocyclic compounds
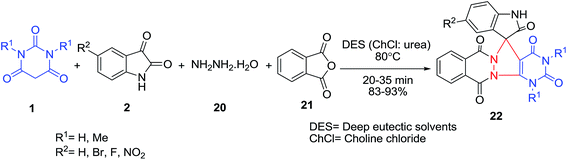
A one-pot four-component domino protocol has been reported for the synthesis of spirooxindoles spiroannulated with pyrazolopyrimidophthalazines 22 involving the reaction of phthalic anhydride 21, hydrazine hydrate 20, isatins 2 and barbituric acid 1 in a deep eutectic solvent (choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea: 1
urea: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Scheme 9).30
2) (Scheme 9).30
3. Synthesis of six-membered heterocycles
3.1. O-Heterocyclic compounds
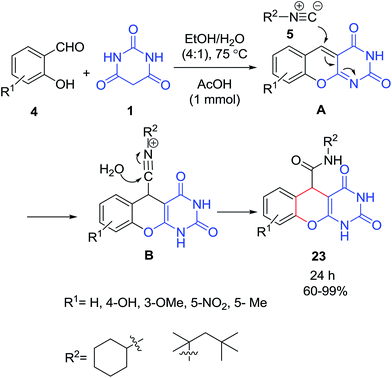
An efficient method for the synthesis of 1H-chromeno[2,3-d]pyrimidine-5-carboxamide derivatives 23 via a one-pot three-component reaction of isocyanide 5, barbituric acid 1 and salicylaldehyde 4 in the presence of acetic acid in ethanol/water mixture was reported (Scheme 10).31 Mechanistically, it is conceivable that the reaction involves the initial formation of the activated alkene (benzopyran ring) A through a Knoevenagel condensation of salicylaldehydes 4 and barbituric acid 1. Benzopyran ring A undergoes nucleophilic addition with the isocyanide 5 followed by nucleophilic attach on the isocyanide by the water to afford 1H-chromeno[2,3-d]pyrimidine-5-carboxamide derivatives 23.One-pot three-component synthesis of 4H-pyrans 26 and spiro-4H-pyrans 27–28 under catalyst-free conditions using glycerol as a promoting medium was reported by Safaei et al. A broad range of substrates including aromatic and heteroaromatic aldehydes 4, isatin derivatives 2, and ninhydrin 25 were condensed with barbituric acid 1 and alkylmalonates 24 (Scheme 11).32 Several catalysts such as tetrabutyl ammonium bromide (TBAB),33 Ni nanoparticles,34 ZnO nano-rods,35 triethylamine under ultrasonic-irradiation,36 meglumine,37 NaHCO3,38 the ionic liquid [H3N+CH2CH2OH][CH3COO−] (HEAA),39 propane-1-sulfonic acid-modified magnetic hydroxyapatite nanoparticles,40 1-butyl-3-methylimidazolium hydroxide ([bmim][OH]),41 SBA-Pr-NH2,42 Mn(bpyo)2/MCM-41,43 silica-supported organocatalyst system based on L-proline (SSLP),44 tetrabutyl ammonium acetate (TBAA),45,46 triethylammonium acetate,47 without any catalysts under microwave irradiation,48 stabilized nickel nanoparticles in ethylene glycol,49 catalyst-free,50 sulfonic acid functionalized SBA-15 (SBA-Pr-SO3H),51 iodine,52 triethylamine,53 oleic acid,54 silica sulfuric acid nanoparticles,55 4-dimethylaminopyridine (DMAP),56 Al-HMS-20,57 ZnO-supported copper oxide,58 dibutylamine (DBA)59 CaHPO4,60 nano-sawdust-OSO3H,61 ZnFe2O4 nanoparticles,62 nano-titania sulfuric acid (TSA) and boric acid [B(OH)3],63 [Amb]-L-prolinate,64 the acidic ionic liquid succinimidinium hydrogensulfate ([H-Suc]HSO4)65 and the dicationic ionic liquid diethylene glycol-bis(3-methylimidazolium) dihydroxide [DiEG(mim)2][OH]2 (ref. 66) have been employed successfully in these reactions.
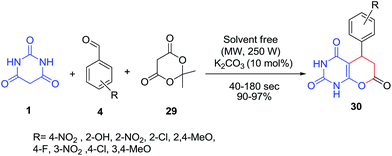
Novel pyrano[2,3-d]pyrimidine-2,4,7-triones 30 were synthesized via a three-component reaction of aromatic aldehydes 4, Meldrum's acid 29 and barbituric acid 1 in the presence of K2CO3 under microwave irradiation (Scheme 12).67 An electrochemical strategy was also described for the synthesis of these products in ethanol in an undivided cell and in the presence of sodium bromide as an electrolyte at room temperature.68
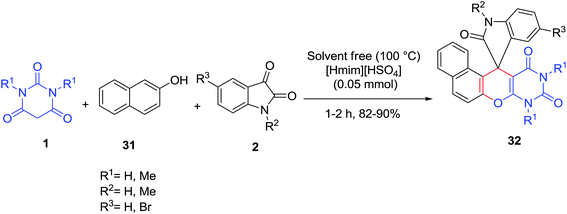
An efficient method has been developed for the synthesis of novel spironaphthopyrano[2,3-d]pyrimidine-5,3′-indolines 32 via a one-pot three-component condensation reaction of barbituric acid derivatives 1, β-naphthol 31 and isatins 2 in the presence of [Hmim][HSO4] as an efficient and reusable catalyst (Scheme 13).69 This reaction was later studied under solvent-free conditions in the presence of SBA-Pr-SO3H as a heterogeneous nanoporous catalyst.70 Replacement of isatin with aldehydes in this reaction has been also investigated and iodine was found to be a highly efficient catalyst for this three-component coupling reaction.71
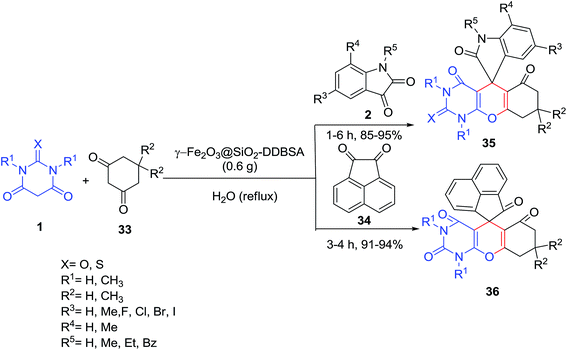
Dodecyl benzenesulfonic acid functionalized silica-coated magnetic nanoparticles (γ-Fe2O3@SiO2-DDBSA) were identified as an efficient catalyst for the synthesis of a library of spirooxindole-pyrimidine derivatives 35–36 by three-component condensation reaction of cyclohexane-1,3-diones 33, barbituric acids 1 and isatins 2 or acenaphthylene-1,2-dione 34 (Scheme 14).72 Alum (KAl(SO4)2·12H2O),73 gluconic acid aqueous solution (GAAS)74 and MnFe2O4@NH2@2AB-Ni nanoparticles75 are other catalysts that were employed in this reaction.
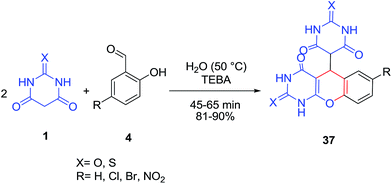
A series of novel 5-(2,3,4,5-tetrahydro-1H-chromeno[2,3-d]pyrimidin-5-yl)pyrimidione derivatives 37 has been synthesized from substituted salicylaldehydes 4 and barbituric acid or 2-thiobarbituric acid 1 in water catalyzed by phase transfer catalysis of triethylbenzyl ammonium chloride (TEBA) (Scheme 15).76 The newly synthesized derivatives exhibited significant in vitro antibacterial activity. I2 (10 mol%) was also applied as catalyst in this reaction.77
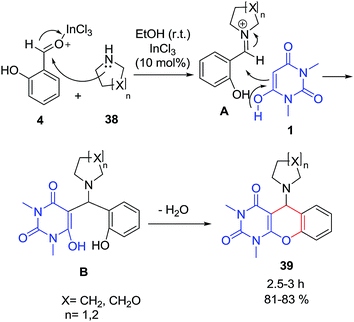
One-pot three component Mannich reaction starting from 1,3-dimethylbarbituric acid 1, salicylaldehyde 4 and different cyclic secondary amines viz pyrrolidine, piperidine and morpholine 38 in the presence of InCl3 as catalyst was reported by Sharma et al. (Scheme 16).78 According to the proposed mechanism, salicylaldehyde 4 reacts with pyrrolidine 38 to give intermediate A which then suffers nucleophilic attack by 1 and gives B. Intermediate B on further elimination of water gives the desired product 39.
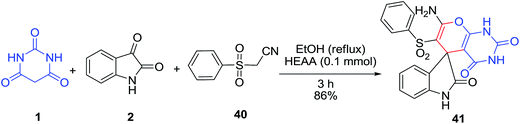
A novel spiro-2-amino-3-phenylsulfonyl-4H-pyran 41 was synthesized via the three component, one pot reaction of phenylsulfonylacetonitrile 40 and barbituric acid 1 with isatin 2 in ethanol using basic ionic liquid 2-hydroxyethyl ammonium acetate [H3N+CH2CH2OH][CH3COO−] (HEAA) as the catalyst (Scheme 17).79
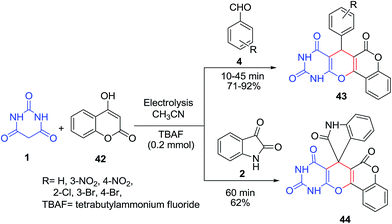
Synthesis of novel chromeno[3′,4′:5,6]pyrano[2,3-d]pyrimidines 43–44 was described using the electrogenerated anion of acetonitrile as the base in the presence of tetrabutylammonium fluoride as an effective supporting electrolyte in a one-pot, three-component condensation of barbituric acid 1, an aromatic aldehyde 4 or isatin 2, and 4-hydroxycoumarin 42 (Scheme 18).80
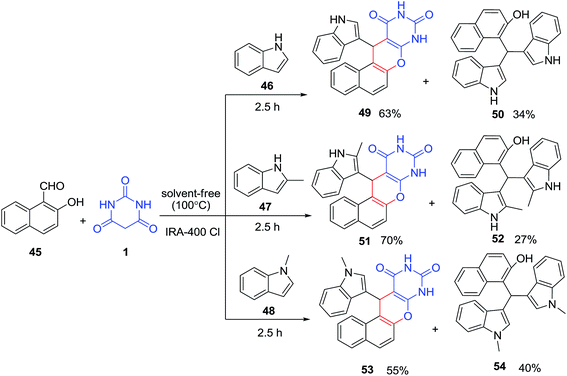
An efficient Amberlite IRA-400 Cl resin catalyzed multicomponent reaction of 2-hydroxy-1-naphthaldehyde 45, barbituric acid 1 and indole derivatives 46–48 under solvent free conditions has been outlined by Harichandran and coworkers (Scheme 19).81 The expected 4H-chromene derivatives 49, 51 and 53 were obtained in moderate to good yields (55–70%) and bis(indolyl) methanes 50, 52 and 54 (27–40%) as minor products.
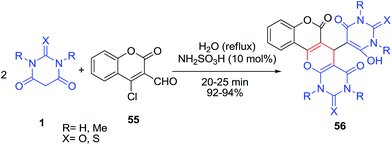
An efficient one-pot procedure for the synthesis of pyranocoumarins 56 by the condensation of 4-chloro-3-formylcoumarin 55 with barbituric acids 1 via Knoevenagel, Michael and cyclization sequences using sulfamic acid as catalyst in aqueous media was reported by Siddiqui (Scheme 20).82
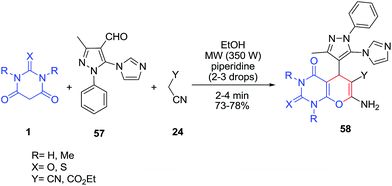
One-pot three-component cyclocondensation reaction of 5-(1H-imidazol-1-yl)-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde 57, barbituric acid 1 and malononitrile/ethylcyanoacetate 24 was successfully carried out in the presence of piperidine as a basic catalyst (Scheme 21).83 All the final motifs have been screened for their preliminary in vitro antibacterial, anti-tuberculosis, and anti-malarial activities.
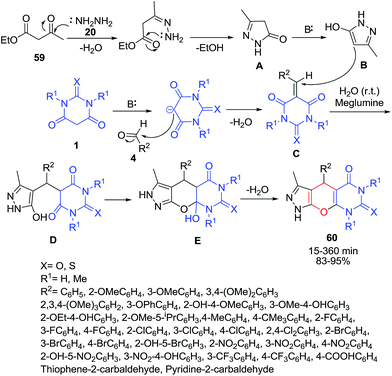
An efficient protocol for the synthesis of pyrazolopyranopyrimidines 60 via one-pot, four-component reaction of ethyl acetoacetate 59, hydrazine hydrate 20, aromatic aldehydes 4 and barbituric acid 1 has been developed using meglumine as catalyst (Scheme 22).84 The mechanism of this reaction appears to involve the formation of 3-methyl-1H-pyrazol-5(4H)-one A by condensation of ethyl acetoacetate and hydrazine, which would be converted to its corresponding enolate form B in the presence of meglumine. The intermediate B is relatively stable compared with other enolates since it is aromatic. The amino group in meglumine plays a major role for the formation of intermediate C from Knoevenagel condensation of benzaldehyde 4 and barbituric acid 1. Subsequently, intermediate B is reacted with Knoevenagel condensate C through Michael addition to produce intermediate D, which underwent intramolecular cyclization by the nucleophilic addition of enolate oxygen to carbonyl group to afford intermediate E. Elimination of water form intermediate E provides target product 60. In another study, application of DABCO as catalyst was investigated in this reaction.85
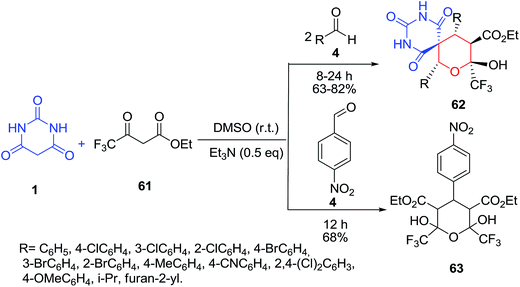
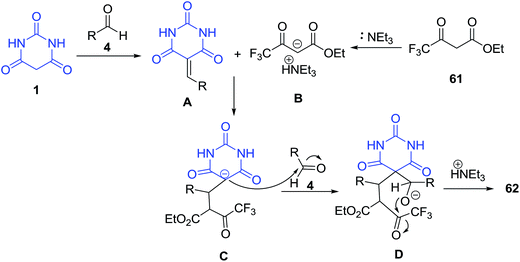
Ethyl-7,11-diaryl-9-hydroxy-1,3,5-trioxo-9-(trifluoromethyl)-8-oxa-2,4-diazaspiro[5.5]undecane-10-carboxylate derivatives 62 were synthesized from barbituric acid 1, aromatic aldehydes 4 and ethyl 4,4,4-trifluoro-3-oxobutanoate 61 via a one-pot, multi-component reaction catalyzed by Et3N (Scheme 23).86 On the other hand, in the case of 4-nitrobenzaldehyde as a reaction substrate, this compound did not give the expected product but diethyl 2,6-dihydroxy-4-nitro-phenyl-2,6-bis-trifluoromethyl-tetrahydro-pyran-3,5-dicarboxylate 63 was obtained under the reaction conditions. In the proposed mechanism for this reaction, first, one molecule of barbituric acid 1 condenses with one molecule of aromatic aldehyde 4 via an initial Knoevenagel condensation reaction, then followed by a Michael addition reaction catalyzed by Et3N, the intermediate C is formed. Intermediate C reacts with the second molecule of aromatic aldehyde 4 to afford the intermediate D, which undergoes the intramolecular cyclization reaction to afford the products 62 (Scheme 24).
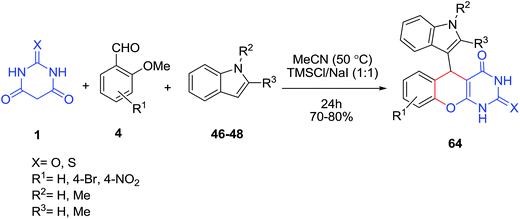
Trimethylsilyl iodide (TMSI), generated in situ from TMSCl and NaI, has been employed as an efficient reagent for the one-pot synthesis of 9-(1H-indol-3-yl)xanthen-4-(9H)-ones 64 through the reaction of 2-methoxybenzaldehydes 4 (as O-methyl protected salicylaldehydes), indoles 46–48 and barbituric acid 1 (Scheme 25).87
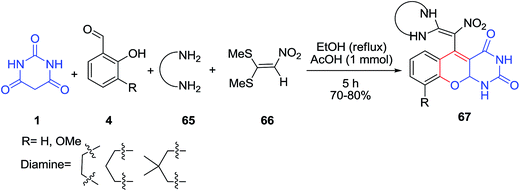
An efficient synthesis of chromeno[2,3-d]pyrimidine-2,4-dione derivatives 67 with a nitroketene-aminal moiety via four-component reaction of salicylaldehydes 4, barbituric acid 1, diamines 65 and 1,1-bis(methylsulfanyl)-2-nitroethene 66 in EtOH in the presence of AcOH was reported (Scheme 26).88
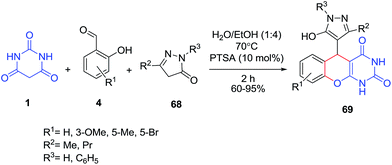
The three-component reactions of salicylaldehydes 4, barbituric acid 1 and pyrazolones 68 in the presence of p-toluenesulfonic acid, have been reported to give pyrazolchromeno[2,3-d]pyrimidine-one derivatives 69 in good yields (Scheme 27).89
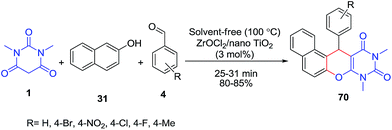
ZrOCl2/nano-TiO2 has been used as an efficient catalyst for the preparation of naphthopyranopyrimidines 70 by the three-component reaction of aldehydes 4, β-naphthol 31 and 1,3-dimethylbarbituric acid 1 under solvent-free conditions (Scheme 28).90
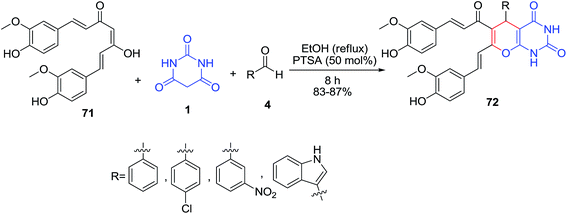
A three component reaction of curcumin 71, barbituric acid 1 and aldehydes 4 in the presence of p-toluenesulfonic acid as catalyst in ethanol (reflux) was reported (Scheme 29).91
3.2. N-Heterocyclic compounds
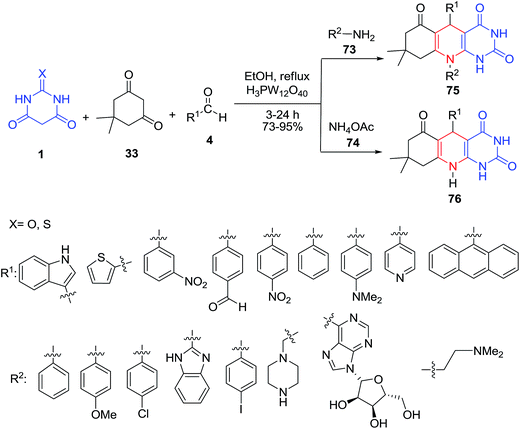
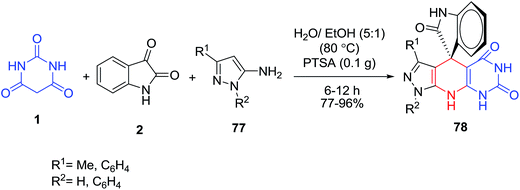
A one-pot method for the efficient synthesis of novel 8,9-dihydro-8,8-dimethyl-5,10-diphenylpyrimido[4,5-b]quinoline-2,4,6(1H,3H,5H,7H,10H)-trione derivatives 75–76 via a four-component condensation reaction of aldehydes 4, amines 73–74, dimedone 33 and barbituric acid 1 in the presence of tungstophosphoric acid (H3PW12O40) as catalyst was described (Scheme 30).92Novel pyrazolopyridine-spiroindolinones 78 were prepared by the three-component reaction of 5-aminopyrazoles 77, isatin 2 and barbituric acid 1 in aqueous media and catalyzed by p-TSA. This protocol provides a simple one-step procedure with the advantages of easy work-up, mild reaction conditions and environmentally benign (Scheme 31).93 Later, applications of the ionic liquid [Bmim]PF6 as the reaction medium and alum as the catalyst,94 SG@NH2@2AB-Sn (a tin complex immobilized on silica gel) in water,95 and sulfamic acid (H2NSO3H) as a green catalyst96 have made it possible to synthesize these heterocyclic compounds. Pyrazolopyridine-spiroindolinones were also obtained by four-component domino reactions from the reaction of phenylhydrazine, 3-aminocrotononitrile, isatin/acenaphthylene-1,2-dione, and barbituric acid in the presence of (±)-camphor-10-sulfonic acid (CSA).97
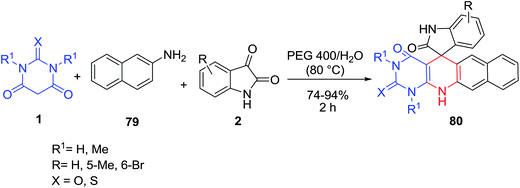
A one-pot three-component process for efficient synthesis of spiro[dihydropyridine-oxindole] scaffolds 80 from the reaction of isatins 2, 2-naphthylamine 79 and barbituric acids 1 in poly(ethylene glycol) 400/H2O under catalyst-free conditions was described (Scheme 32).98 Iodine has been also used as a catalyst in this three-component reaction in ethanol media.99
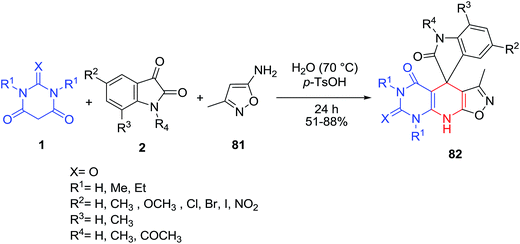
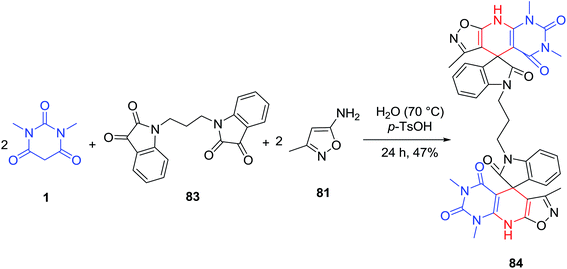
A one-pot three-component condensation reaction of isatins 2, 5-amino-3-methyl isoxazole 81 and barbituric acids 1 to give spiro[indolin-isoxazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine]triones 82 using a catalytic amount of p-toluenesulfonic acid in water was described by Rahmati and Khalesi (Scheme 33).100 Another feature of this reaction was revealed when 5-amino-3-methyl isoxazole 81 (2 equiv.) and 1,3-dimethyl barbituric acid 1 (2 equiv.) were simultaneously added to 1,1′-(propane-1,3-diyl)bis-isatins 83. The reaction proceeded as a pseudo five-component reaction and resulted in the synthesis of spiro[indoline-isoxazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine]triones 84 (Scheme 34).
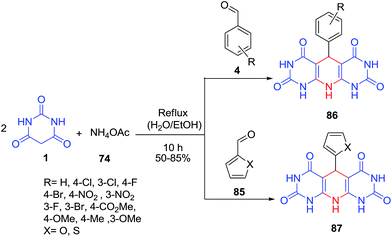
A green procedure has been developed for the synthesis of dihydropyridine derivatives 86–87 by a simple condensation of barbituric acids 1, different aldehydes 4, 85 and ammonium acetate 74 in water under catalyst-free conditions (Scheme 35).101 Excellent yields and purity were obtained with only filtration and washing with hot water and ethanol.
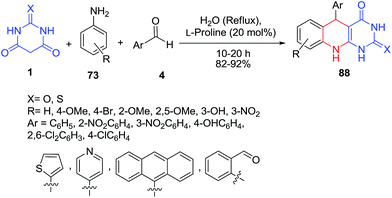
A green one-pot process to achieve 5-aryl-pyrimido[4,5-b]quinoline derivatives 88, using a three-component reaction involving anilines 73, aldehydes 4 and barbituric acids 1 was developed (Scheme 36).102 This protocol was accomplished efficiently using L-proline as catalyst in an aqueous medium to give the corresponding products in high yield. The multicomponent reactions showed good regioselectivity and computational studies were used to investigate the selectivity. Mosslemin and coworkers have reported this reaction catalyzed by 1,4-diaza-bicyclo[2.2.2]octane (DABCO) in water.103 Using microwave heating, reaction times were shortened from 12 h to under a minute and yields were generally higher.
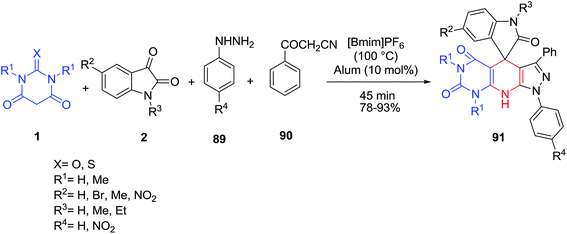
Ghahremanzadeh and co-workers reported a four-component reaction of barbituric acid derivatives 1, isatins 2, phenyl hydrazines 89 and 3-oxo-3-phenylpropanenitrile 90 in the presence of alum using the ionic liquid [Bmim]PF6 as an effective green reaction medium (Scheme 37).104
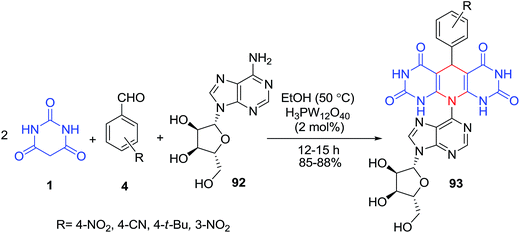
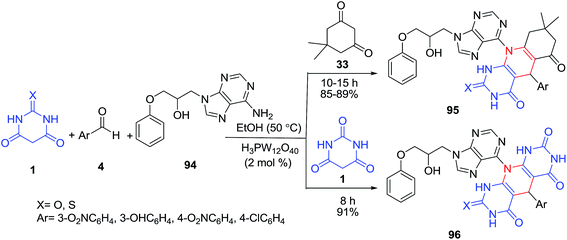
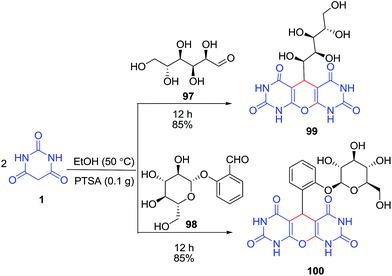
An efficient protocol for the synthesis of some new pyrimidine-fused nucleoside analogues 93, using a pseudo four-component coupling reaction of nucleosides 92, aldehydes 4 and barbituric acid derivatives 1 was developed (Scheme 38).105 The four-component coupling reaction of nucleoside 94, dimedone 33, barbituric acid/2-thiobarbituric acid 1 and various aldehydes 4 under the optimized conditions was also examined (Scheme 39). In another study, the same group of authors reported the pseudo multicomponent reaction of barbituric acid 1 and two different aldehydes 97–98 (Scheme 40).106
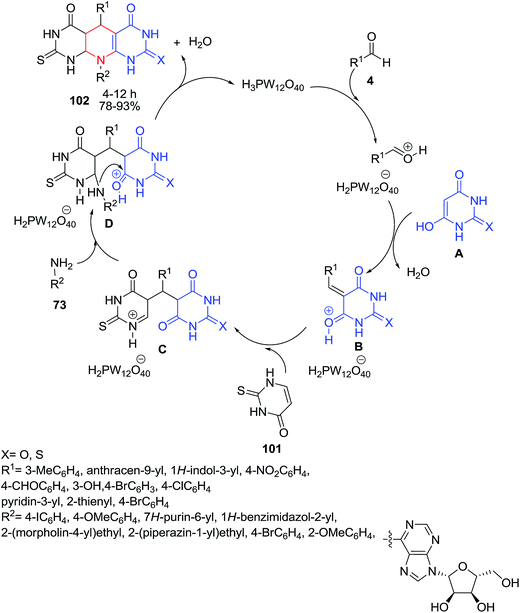
In a similar study, an efficient multicomponent approach for the preparation of new pyrimidine-fused scaffolds 102, via a four-component reaction of aldehydes 4, amines 73, barbituric acids 1 and thiouracil 101 was reported by the same group (Scheme 41).107 In the proposed mechanism, barbituric acid 1 in its enol form A reacts first with the activated aldehyde to form the corresponding 5-(arylmethylidene)barbiturate B, and then thiouracil 101 adds to this adduct to produce the intermediate C. The latter can undergo a reaction with the amine component 73, generating D, which is converted to the desired product 102 by an intramolecular condensation reaction.
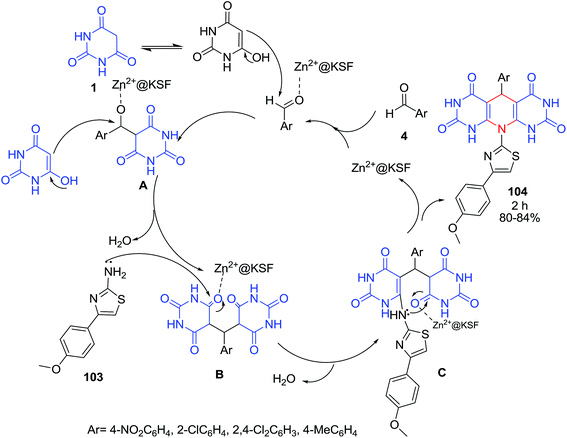
Mahmoodi and co-workers reported the one-pot pseudo four-component reaction of aldehydes 4, barbituric acid 1 and thiazole 103 using Zn2+@KSF under reflux condition (Scheme 42).108 According to proposed mechanism, nucleophilic addition of barbituric acid 1 to activated aldehyde in the presence of catalyst led to intermediate A which becomes involved in the addition reaction with second molecule of 1 and subsequent addition of thiazole 103 to adduct B via C by losing water and finally further intramolecular cyclization led to 104. The antibacterial activities of compounds were also screened.
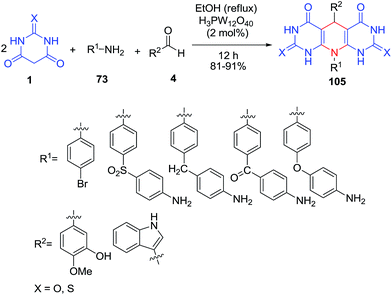
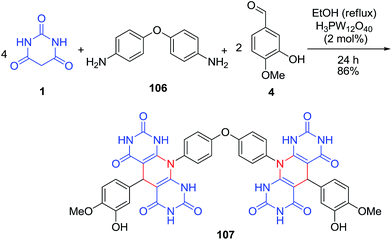
The synthesis of a set of pyrimidine-fused heterocycles 105 via the treatment of barbituric acid 1 with amines 73 and aldehydes 4 allowed the discovery of new ligands with modest and selective inhibitory activity (Scheme 43).109 In the same paper, for synthesis of compound 107, a mixture of barbituric acid 1, isovanillin 106, aldehyde 4 and H3PW12O4 were refluxed in EtOH (Scheme 44). Compound 107 with a two pyrimidine-fused heterocycle moieties showed a significant ability for inhibition of mouse α-Gls. A p-toluenesulfonic acid-catalyzed reaction was also reported by Memarzadeh et al.110
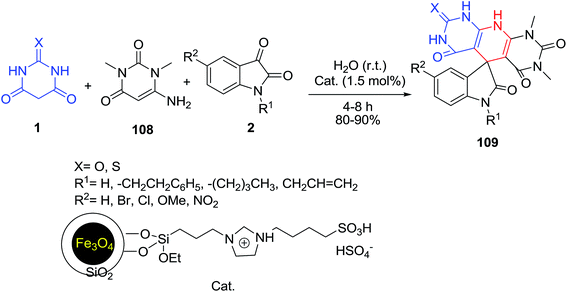
One-pot synthesis of novel spirooxindole derivatives 109 from substituted isatins 2, 6-amino-1,3-dimethyluracil 108 and barbituric acid/thiobarbituric acid 1 was investigated in the presence of magnetic, supported, acidic ionic liquid as an efficient catalyst (Scheme 45).111
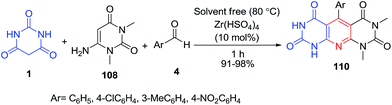
A three-component reaction of aromatic aldehydes 4, 6-amino-1,3-dimethyluracil 108 and barbituric acid 1 in the presence of Zr(HSO4)4 as a heterogeneous catalyst, under solvent-free conditions brought an efficient method for the preparation of pyrimido[5′,4′:5,6]pyrido[2,3-d]pyrimidines 110 (Scheme 46).112 In another studies, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)113 and SBA-Pr-SO3H114 have been reported as efficient catalysts in this reaction.
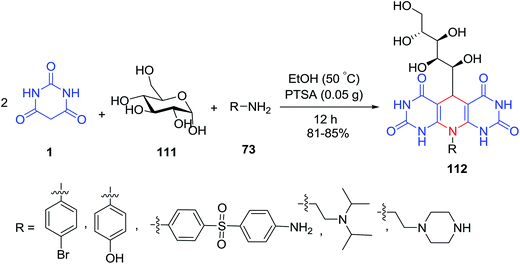
Yousefi et al. introduced an efficient multicomponent approach for the preparation of 10-substituted-5-(1,2,3,4,5-pentahydroxypentyl)dihydro[2,3-d:6,5-d] dipyrimidine-tetraones 112. According to this protocol, when (+)-D-glucose 111 and amines 73, reacted with two equivalent of barbituric acid 1 in the presence of p-toluenesulfonic acid as a catalyst, the excellent yield of products were obtained (Scheme 47).115 In a similar study, the same group of authors have investigated the three-component reaction of sugar, barbituric acid, and 4,4′-oxydianiline in the presence of p-toluenesulfonic acid in EtOH.116
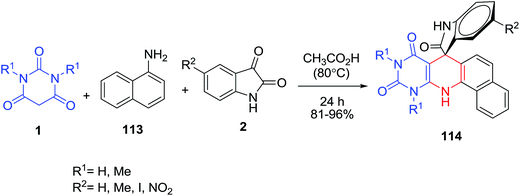
Rahmati and Eskandari-Vashareh have reported an efficient one-pot synthesis of a new series of spiro[benzo[h]quinoline-7,3′-indolines] 114 by the reaction of isatins 2, 1-naphthylamine 113 and barbituric acid 1 in acetic acid (Scheme 48).117
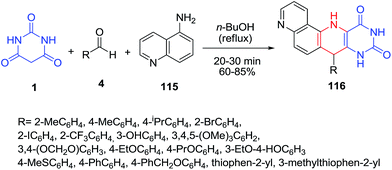
New pyrimidine-fused 1,7-phenanthroline derivatives 116 have been synthesized by three-component condensation of barbituric acid 1 with quinolin-5-amine 115 and aromatic or heteroaromatic aldehydes 4 (Scheme 49).118
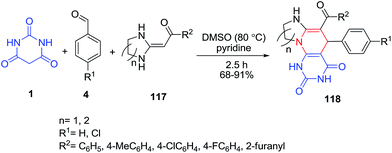
Polycyclic fused ring quinazoline-2,4-dione derivatives 118 were synthesized in a one-pot three component cascade protocol by the reaction of heterocyclic ketene aminals 117, barbituric acid 1 and benzaldehydes 4 (Scheme 50).119
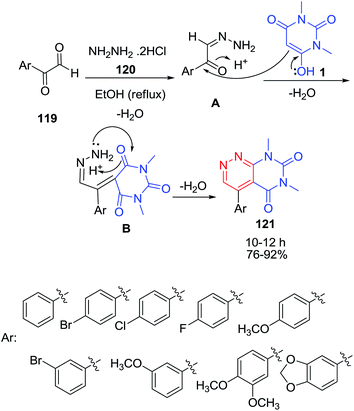
A series of new 4-aryl-6,8-dimethylpyrimido[4,5-c]pyridazine-5,7(6H,8H)-diones 121 have been synthesized via three component reaction of 1,3-dimethylbarbituric acid 1 with arylglyoxals 119 in the presence of hydrazinium dihydrochloride 120 in ethanol (Scheme 51).120 All compounds were evaluated as monoamine oxidase inhibitors. The suggested mechanism for the formation of 121 involves the initial formation of arylglyoxal monohydrazone A through a simple imine condensation reaction. The subsequent knoevenagel condensation of 1,3-dimethyl barbituric acid enolic form with the aryl monohydrazone carbonyl leads to the formation of the intermediate B. Progress of the reaction via fulfillment of an intramolecular imine condensation in the intermediate B gave the final product 121.
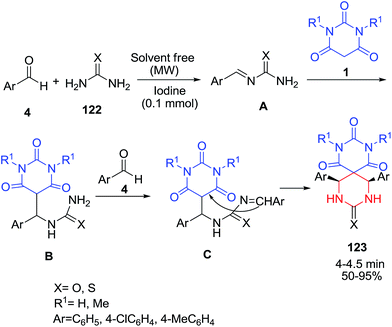
A Biginelli-type reaction of aldehydes 4, urea or thiourea 122 and barbituric acids 1 was performed using molecular iodine as a mild Lewis acid catalyst under microwave irradiation in solvent-free conditions for the synthesis of various symmetrical spiro heterobicyclic compounds 123 (Scheme 52).121 Although the mechanism of this reaction has not been experimentally elucidated, it is likely that N-acylimine species A is formed first by the reaction of urea 122 and aldehyde 4 via a nucleophilic addition and dehydration. Subsequent addition of the iminium ion to barbituric acid derivatives 1 in presence of iodine catalyst produces an open chain ureide B. The second molecule of aldehyde 4 is then added to the ureide B to furnish intermediate C, which subsequently cyclizes to provide spiro heterobicyclic ring 123. Alternatively, aldehyde and barbituric acid may react via a Knoevenagel condensation reaction, followed by a Michael type reaction with urea to produce ureide B. This then reacts with a second molecule of aldehyde, which ultimately cyclizes to the desired product. This reaction was also investigated in the presence of sulfonic acid functionalized nanoporous silica SBA-Pr-SO3H.122
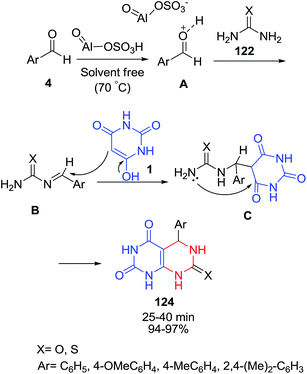
Nasr-Esfahani and Taei have reported the one-pot reaction between aldehyde 4, urea or thiourea 122 and barbituric acid 1 under solvent-free conditions in the presence of aluminatesulfonic acid nanoparticles (ASA NPs) as a heterogeneous solid acid catalyst (Scheme 53).123 The proposed mechanism includes the acid-catalyzed formation of C–N bond from the benzaldehyde and urea. It was indicated that Biginelli reaction catalyzed by ASA NPs proceeds predominately through arylidene-urea intermediate B. In fact, ASA NPs is a solid proton source that its solid surface attracts the substrate molecules and then protonates their active sites to be ready for reaction. In another report, triethyl benzyl ammonium chloride (TEBAC) was used as a catalyst in this reaction to afford pyrimido[4,5-d]pyrimidines 124 under solvent-free conditions.124
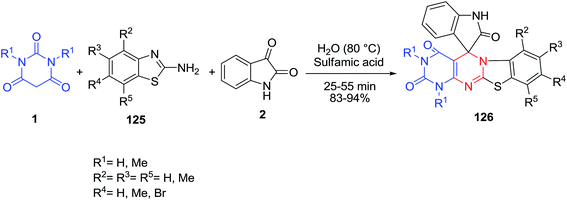
Spiro[benzothiazolopyrimidopyrimidine-indoline]diones 126 have been synthesized by sulfamic acid-catalyzed multicomponent domino reaction of 2-aminobenzothiazoles 125 with isatin 2 and barbituric acid 1 in an aqueous medium (Scheme 54).125
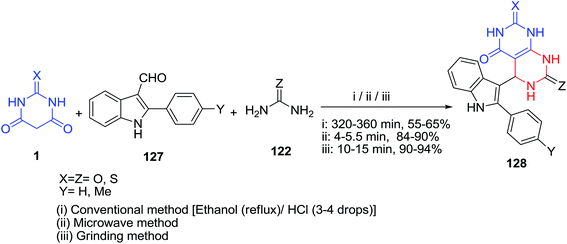
A series of 5-indolylpyrimido[4,5-d]pyrimidinones 128 were obtained by multi-component reaction of 3-formylindole 127, thiobarbituric acid/barbituric acid 1 and thiourea/urea 122. In this regard, two simple and eco-friendly synthetic protocols including solid supported microwave irradiation and grindstone technology were developed which provide higher yields in shorter reaction time (Scheme 55).126 Representative compounds were also evaluated for their antimicrobial activities and some of them showed promising results.
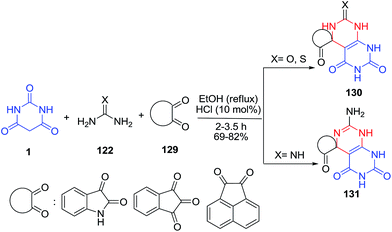
Some highly functionalized novel heterocyclic compounds containing spiro[quinazoline/pyrimidine]ones 130–131 were synthesized by the reaction of isatin (acenaphthylene-1,2-dione/ninhydrin) 129, barbituric acid 1 and urea/thiourea or guanidine 122 (Scheme 56).127 It is noteworthy that one C–C and two C–N bonds were formed with consequent creation of spiro derivatives in these one-pot three-component processes.
4. Synthesis of 5-monosubstituted barbiturates
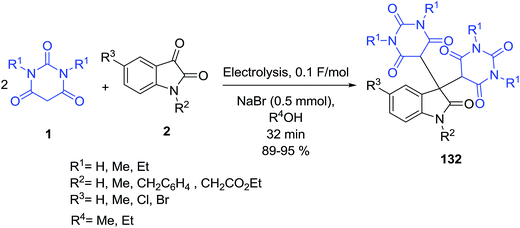
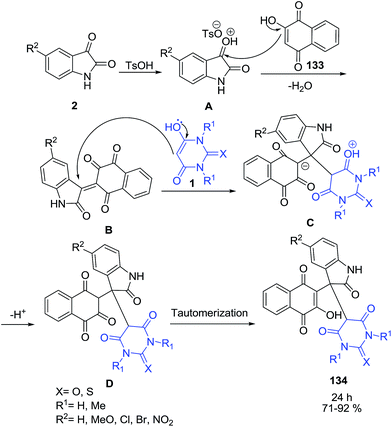
Electrocatalytic transformation of isatins 2 and barbituric acids 1 in ethanol in an undivided cell in the presence of sodium bromide as an electrolyte results in the formation of substituted 5,5′-(2-oxo-2,3-dihydro-1H-indole-3,3-diyl)bis(pyrimidine-2,4,6(1H,3H,5H)-triones) 132 in 89–95% yield (Scheme 57).128 This developed efficient electrocatalytic approach to the target compounds was 10 times faster than general chemical method.An efficient method has been developed for the synthesis of a novel series of unsymmetrical 3,3-disubstituted oxindoles 134 in good-to-high yields by a one-pot three-component condensation reaction of 2-hydroxynaphthalene-1,4-dione 133, isatins 2 and barbituric acid derivative 1 using p-toluenesulfonic acid as a catalyst (Scheme 58).129 In a first step, the isatin C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group is activated by TsOH. Then, reaction between 133 and compound A gives α,β-unsaturated compound B. Michael addition of barbituric acid 1 to B gives intermediate C. Subsequent H+-transfer leads to compound D, which undergoes tautomerization to product 134.
O group is activated by TsOH. Then, reaction between 133 and compound A gives α,β-unsaturated compound B. Michael addition of barbituric acid 1 to B gives intermediate C. Subsequent H+-transfer leads to compound D, which undergoes tautomerization to product 134.
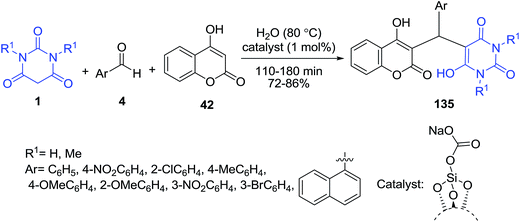
Catalytic condensation between barbituric acid derivatives 1, aryl aldehydes 4 and 4-hydroxycoumarin 42 in the presence of silica sodium carbonate (SSC) as a catalyst has been developed by Eskandari and coworkers to access the corresponding benzylbarbiturocoumarin derivatives 135 (Scheme 59).130
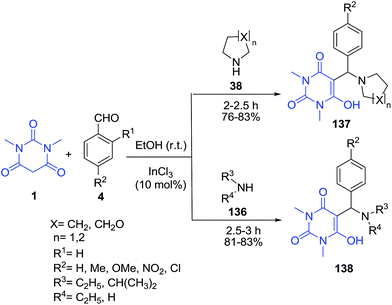
Some novel 5-aminoalkylbarbituric acids 137–138 have been synthesized from a one-pot three component Mannich reaction of 1,3-dimethyl barbituric acid 1, aryl aldehyde 4 and amines 38 and 136 (Scheme 60).78
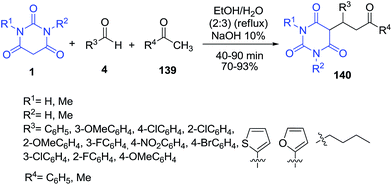
Synthesis of 5-monoalkylbarbiturates 140 through NaOH catalyzed one-pot reaction of barbituric acids 1, aldehydes 4 and ketones 139, involving a domino aldol-Michael reaction was described by Kalita et al. (Scheme 61).131
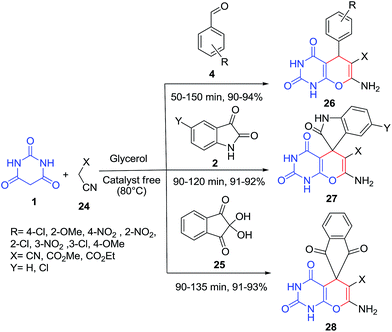
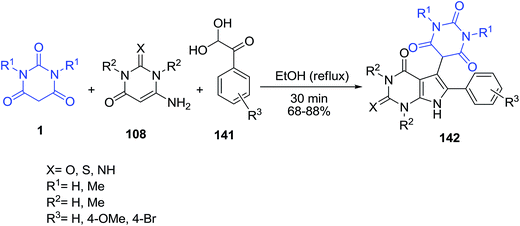
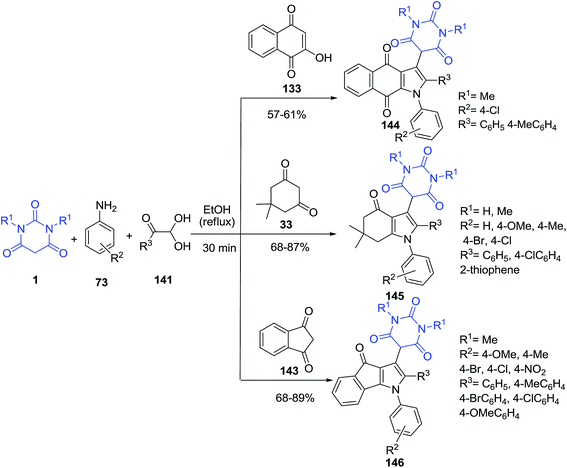
An efficient method for the synthesis of pyrimidine functionalized pyrrolo-annelated derivatives 142, 144–146 has been developed via a catalyst-free, one-pot three component reaction of barbituric acid 1, aminouracil 108 and phenylglyoxal hydrate derivatives 141 (Scheme 62) and four component reaction of 1,3-indanedione 143/dimedon 33/2-hydroxynaphthalene-1,4-dione 133, barbituric acid 1, aromatic amine 73 and phenylglyoxal hydrate 141 (Scheme 63).132
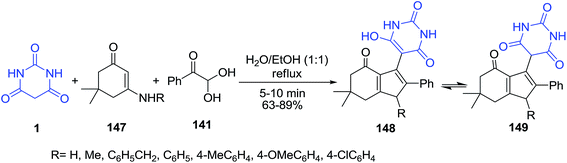
Three-component condensations reaction of barbituric acid 1, cyclic enamino ketone 147 and phenylglyoxal hydrate 141 under reflux condition has been examined (Scheme 64).133 Compounds 148 can also exist as tautomers 149.
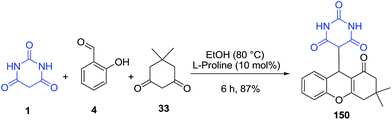
Three-component reactions of salicylaldehydes 4, dimedone 33 and barbituric acid 1 were developed using L-proline as a catalyst to generate 4H-chromene derivatives 150 in good to excellent yields (Scheme 65).134 The reactions were performed in ethanol under mild and metal-free conditions. In a similar study, this reaction was developed using tendon hydrolysate (TH) as a catalyst.135 Non-catalytic three-component reaction was also reported resulted in the formation of related products in 90–95% yields.136
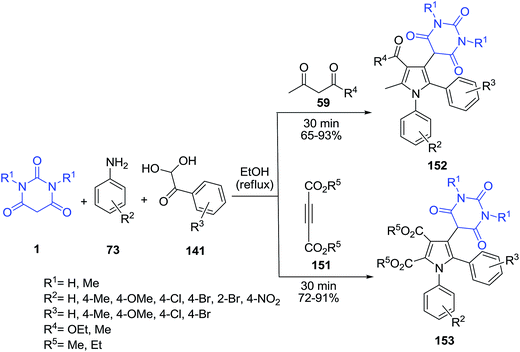
A catalyst-free chemical protocol for the construction of pyrimidine containing poly-functionalized pyrroles 152–153 from a four-component domino reaction of acyclic-1,3-dicarbonyls 59 or electron deficient alkynes 151, aromatic amines 73, barbituric acid 1 and arylglyoxal hydrates 141 under mild reaction conditions has been developed (Scheme 66).137
5. Synthesis of Knoevenagel adducts
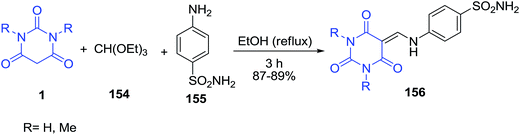
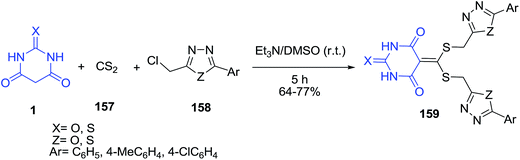
A three-component reaction of sulfanilamide 155, barbituric acid 1 and triethyl[orthoformate] 154 in refluxing ethanol constituted an efficient one-pot procedure for the synthesis of 1,3-dicarbonyl substituted methylaminobenzene-sulfonamide derivatives 156 (Scheme 67).138 The inhibitory effects of the compounds on the activity of purified human carbonic anhydrase (hCA) I and hCA II were evaluated.A new class of trisheterocyclic systems, bisoxadiazolyl/bisthiadiazolyl pyrimidinetriones/thioxopyrimidinediones 159 was prepared by the condensation of barbituric acid derivatives 1, carbon disulfide 157 and 1,3,4-oxadiazole/thiadiazole 158 under base catalysis (Scheme 68).139 The antimicrobial activity of the synthesized compounds was studied and the results revealed that compounds having thioxopyrimidinedione in combination with bisthiadiazole unit exhibited high activity.
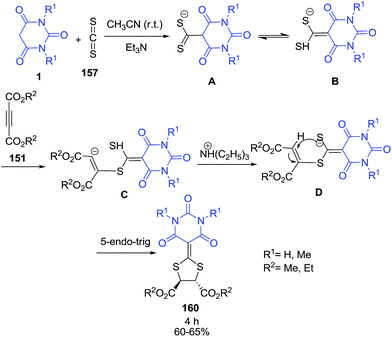
A series of ketene dithioacetals 160 has been synthesized by condensation of barbituric acid 1 with carbon disulfide 157 and dialkyl acetylenedicarboxylates 151 in the presence of triethylamine (Scheme 69).140 The reaction of barbituric acid 1 with CS2 157 in the presence of triethylamine gave the intermediates A and B as thioketone and enethiol tautomers. After the addition of dialkyl acetylenedicarboxylates 151, the formation of intermediate C occurred via Michael addition reaction of the sulfur anion at the sp carbon atom of dialkyl acetylenedicarboxylates. Then, intramolecular hydrogen transfer occurred which produced intermediate D. Finally, intermediate D undergoes a second Michael addition via a 5-endo-trig intramolecular cyclization to form product 160.
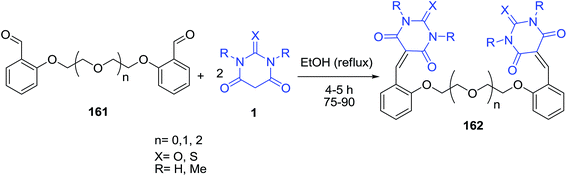
Knoevenagel condensation reaction of ethylene glycol-based aromatic aldehyde 161 with barbituric acid derivatives 1 in ethanol produced novel benzylidene barbituric acid derivatives 162 (Scheme 70).141
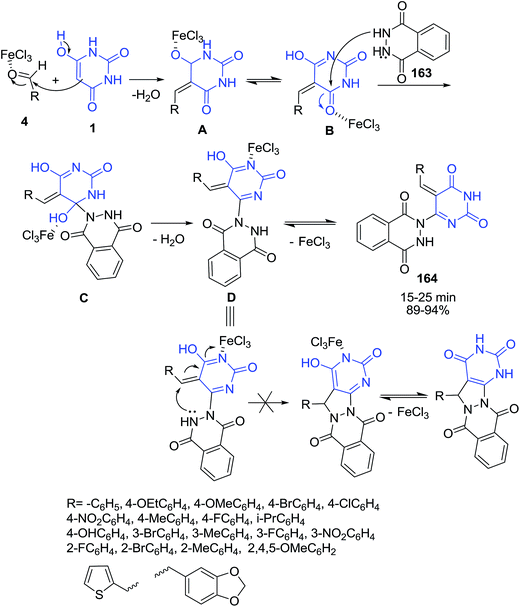
Three-component coupling of phthalhydrazide 163, aldehydes 4 and barbituric acid 1 has been accomplished in the presence of FeCl3 under solvent-free conditions to afford the corresponding (tetrahydro-2,6-dioxopyrimidin-4-yl)-2,3-dihydrophthalazine-1,4-dione derivatives 164 in excellent yields (Scheme 71).142 The multicomponent reaction should be proceeding in a stepwise manner. At first, the reaction occurs via Knoevenagel condensation between enolic form of barbituric acid 1 and aldehyde 4 in the presence of FeCl3 catalyst to form the intermediate, A. This is enolising as B and transforms immediately into one more intermediate, C, by Michael addition of phthalhydrazide 163 at conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of B. Finally, the products obtained by elimination of water molecule in good yields rather than the cyclization products. In another study, Biabangard and Shaterian have reported the synthesis of same compounds 164 via four-component reaction of hydrazine hydrate, phthalic anhydride, aromatic aldehydes and barbituric acid using vitamin B1 supported on alumina (VB1–Al2O3) as a catalyst.143
O bond of B. Finally, the products obtained by elimination of water molecule in good yields rather than the cyclization products. In another study, Biabangard and Shaterian have reported the synthesis of same compounds 164 via four-component reaction of hydrazine hydrate, phthalic anhydride, aromatic aldehydes and barbituric acid using vitamin B1 supported on alumina (VB1–Al2O3) as a catalyst.143
6. Synthesis of zwitterion products
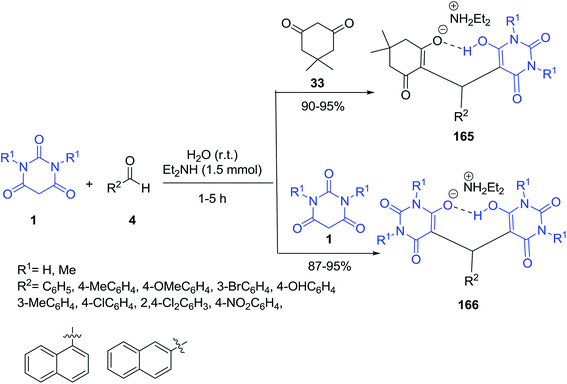
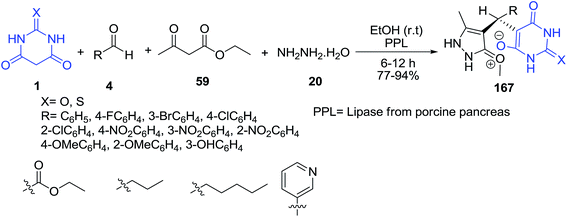
A variety of zwitterion products 165 were obtained by one-pot three-component reaction of barbituric acid 1, aldehydes 4 and dimedone 33 in water in the presence of NHEt2. Also a pseudo three-component reaction was considered in the presence of barbituric acid 1 and aldehyde derivatives 4 (Scheme 72).144 The compounds were subjected to biological evaluation to determine antioxidant activity and inhibition of NO production.An efficient enzymatic four-component reaction of ethyl acetoacetate 59, hydrazine hydrate 20, aldehyde 4 and barbituric acid 1 has been reported for the synthesis of potentially lipophilic zwitterionic 5-monosubstituted barbiturates 167 in ethanol at room temperature and the lipase from porcine pancreas (PPL) found to give optimum efficiency in this reaction (Scheme 73).145 Interestingly, the authors could not detect any formation of the pyrazolopyranopyrimidine throughout the investigation.
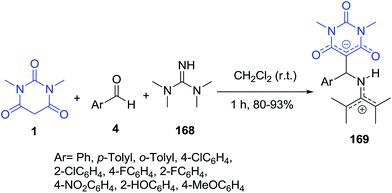
The synthesis of stable charge-separated tetramethylguanidinium-barbituric acid zwitterionic salts 169 was developed through a one-pot three-component reaction of N,N′-dimethylbarbituric acid 1, benzaldehydes 4 and N,N,N′,N′- tetramethylguanidine 168 in CH2Cl2 at room temperature (Scheme 74).146 A dynamic effect was observed in the 1H NMR spectrum of compounds as a result of restricted rotation around the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the guanidinium moiety.
C bond of the guanidinium moiety.
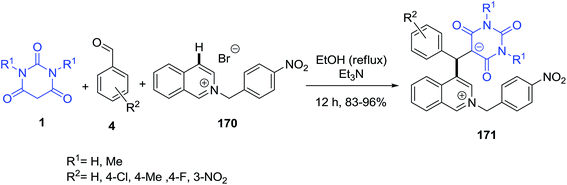
Isoquinolinium zwitterionic salts 171 with an unusual C-4 substitution pattern were efficiently prepared via the multicomponent reaction of in situ formed N-benzylisoquinolinium bromide 170 with aromatic aldehydes 4 and barbituric acid 1 (Scheme 75).147
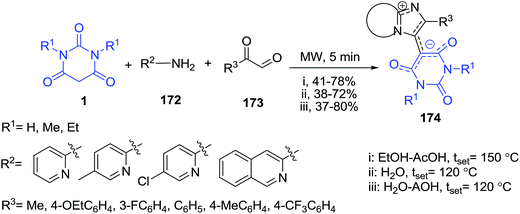
A three-component reaction of 2-aminopyridines/3-aminoisoquinoline 172, 2-oxoaldehyde 173 and barbituric acid derivatives 1 providing access toward a novel class of imidazo[1,2-a]pyridines 174 was studied (Scheme 76).148 The scope of the process was thoroughly explored under three different microwave conditions resulting in the generation of a small library of title compounds. The X-ray data suggested that the obtained products are isolated as mono-hydrates and in solid state exist in a zwitterion tautomeric form.
7. Conclusion
Barbituric acid constitutes the backbone of a large number of interesting compounds. Herein, we surveyed the last 5 year progress on the construction of different types of compounds derived from barbituric acid with typical examples. There is a wide range of multicomponent reactions that include barbituric acid in the synthesis of various organic compounds. This review aims to show representative examples of these multicomponent reactions in recent years. We can still expect many further developments of this compound in synthetic chemistry.Acknowledgements
We are grateful for financial support from the Research Council of Alzahra University.References
- A. Baeyer, Justus Liebigs Ann. Chem., 1864, 130, 129–175 CrossRef
.
- H. Shonle and A. Moment, J. Am. Chem. Soc., 1923, 45, 243–249 CrossRef CAS
.
- C. Nielsen, J. A. Higgins and H. C. Spruth, J. Pharmacol. Exp. Ther., 1925, 26, 371–383 CAS
.
- C. L. Kliethermes, P. Metten, J. K. Belknap, K. J. Buck and J. C. Crabbe, Brain Res., 2004, 1009, 17–25 CrossRef CAS PubMed
.
- P. R. Andrews, G. P. Jones and D. Lodge, Eur. J. Pharmacol., 1979, 55, 115–120 CrossRef CAS PubMed
.
- Archana, V. K. Srivastava and A. Kumar, Bioorg. Med. Chem., 2004, 12, 1257–1264 CrossRef CAS PubMed
.
- B. D. Dhorajiya, B. Z. Dholakiya and R. M. Mohareb, Med. Chem. Res., 2014, 23, 3941–3952 CrossRef CAS
.
- F. Sandberg, Acta Physiol. Scand., 1951, 24, 7–26 CrossRef CAS PubMed
.
- P. Singh, M. Kaur and P. Verma, Bioorg. Med. Chem. Lett., 2009, 19, 3054–3058 CrossRef CAS PubMed
.
- O. M. Ashour, F. N. Naguib, M. M. Khalifa, M. H. Abdel-Raheem, R. P. Panzica and M. H. el Kouni, Cancer Res., 1995, 55, 1092–1098 CAS
.
- V. J. Brown and D. S. Tait, in Behavioral Flexibility: Attentional Shifting, Rule Switching, and Response Reversal, ed. I. P. Stolerman and L. H. Price, Springer Berlin Heidelberg, Berlin, Heidelberg, 2015, pp. 264–269 Search PubMed
.
- N. B. Ilangaratne, N. N. Mannakkara, G. S. Bell and J. W. Sander, Bull. W. H. O., 2012, 90, 871–871A CrossRef PubMed
.
- A. Glover, H. G. Chun, L. M. Kleinman, D. A. Cooney, J. Plowman, C. K. Grieshaber, L. Malspeis and B. Leyland-Jones, Invest. New Drugs, 1987, 5, 137–143 CrossRef CAS PubMed
.
- R. A. Irgashev, G. A. Kim, G. L. Rusinov and V. N. Charushin, ARKIVOC, 2014,(v), 123–131 Search PubMed
.
- J. T. Bojarski, J. L. Mokrosz, H. J. Bartoń and M. H. Paluchowska, in Recent Progress in Barbituric Acid Chemistry, ed. R. K. Alan, Academic Press, 1985, vol. 38, pp. 229–297 Search PubMed
.
- R. M. Shaker and E. A. Ishak, Z. Naturforsch., B: J. Chem. Sci., 2011, 66, 1189–1201 CrossRef CAS
.
- K. T. Mahmudov, M. N. Kopylovich, A. M. Maharramov, M. M. Kurbanova, A. V. Gurbanov and A. J. L. Pombeiro, Coord. Chem. Rev., 2014, 265, 1–37 CrossRef CAS
.
- A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed
.
- A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef PubMed
.
- M. N. Elinson, V. M. Merkulova, A. I. Ilovaisky and G. I. Nikishin, J. Heterocycl. Chem., 2013, 50, 1236–1241 CAS
.
- M. T. Maghsoodlou, G. Marandi, N. Hazeri, S. M. Habibi-Khorassani and A. A. Mirzaei, Mol. Diversity, 2011, 15, 227–231 CrossRef CAS PubMed
.
- A. N. Vereshchagin, M. N. Elinson, E. O. Dorofeeva, T. A. Zaimovskaya, N. O. Stepanov, S. V. Gorbunov, P. A. Belyakov and G. I. Nikishin, Tetrahedron, 2012, 68, 1198–1206 CrossRef CAS
.
- M. B. Teimouri, P. Akbari-Moghaddam and M. Motaghinezhad, Tetrahedron, 2013, 69, 6804–6809 CrossRef CAS
.
- Y. Hosseini, S. Rastgar, Z. Heren, O. Büyükgüngörc and N. N. Pesyan, J. Chin. Chem. Soc., 2011, 58, 309–318 CrossRef CAS
.
- M. Jalilzadeh and N. N. Pesyan, Bull. Korean Chem. Soc., 2012, 43, 3382–3388 Search PubMed
.
- M. B. Teimouri and M. Eskandari, J. Chem. Res., 2011, 35, 500–505 CrossRef CAS
.
- N. Noroozi Pesyan and M. Jalilzadeh, Turk. J. Chem., 2012, 36, 788–804 CAS
.
- S. Ahadi, P. Mirzaei and A. Bazgir, Comb. Chem. High Throughput Screening, 2013, 16, 435–440 CrossRef CAS PubMed
.
- P. Sambavekara, M. Aitawadea, G. Kolekarb, M. Deshmukhb and P. Anbhule, Indian J. Chem., 2014, 53, 1454–1461 Search PubMed
.
- A. Rajawat, S. Khandelwal and M. Kumar, RSC Adv., 2014, 4, 5105–5112 RSC
.
- E. Soleimani, S. Ghorbani and H. R. Ghasempour, Tetrahedron, 2013, 69, 8511–8515 CrossRef CAS
.
- H. R. Safaei, M. Shekouhy, S. Rahmanpur and A. Shirinfeshan, Green Chem., 2012, 14, 1696–1704 RSC
.
- A. Mobinikhaledi, N. Foroughifar and M. A. B. Fard, Synth. Commun., 2011, 41, 441–450 CrossRef CAS
.
- J. M. Khurana and S. Yadav, Aust. J. Chem., 2012, 65, 314–319 CrossRef CAS
.
- M. Hosseini-Sarvari and M. Tavakolian, Comb. Chem. High Throughput Screening, 2012, 15, 826–834 CrossRef CAS PubMed
.
- D. Azarifar, R. Nejat-Yami, F. Sameri and Z. Akrami, Lett. Org. Chem., 2012, 9, 435–439 CrossRef CAS
.
- R.-Y. Guo, Z.-M. An, L.-P. Mo, R.-Z. Wang, H.-X. Liu, S.-X. Wang and Z.-H. Zhang, ACS Comb. Sci., 2013, 15, 557–563 CrossRef CAS PubMed
.
- Y. He, H. Guo and J. Tian, J. Chem. Res., 2011, 35, 528–530 CrossRef CAS
.
- S.-S. Jin, M.-H. Ding and H.-Y. Guo, Heterocycl. Commun., 2013, 19, 139–143 CrossRef CAS
.
- L. Jalili-Baleh, N. Mohammadi, M. Khoobi, L. Ma'mani, A. Foroumadi and A. Shafiee, Helv. Chim. Acta, 2013, 96, 1601–1609 CrossRef CAS
.
- S.-S. Jin and H.-Y. Guo, J. Chem. Res., 2012, 36, 638–640 CrossRef CAS
.
- P. Hajiabbasi, G. Mohammadi Ziarani, A. Badiei and A. A. Soorki, J. Iran. Chem. Soc., 2015, 12, 57–65 CrossRef CAS
.
- M. Daraie, Y. S. Beheshtiha and M. M. Heravi, Monatsh. Chem., 2015, 146, 191–198 CrossRef CAS
.
- A. Khalafi-Nezhad, E. S. Shahidzadeh, S. Sarikhani and F. Panahi, J. Mol. Catal. A: Chem., 2013, 379, 1–8 CrossRef CAS
.
- S. Riyaz, A. Indrasena, A. Naidu and P. Dubey, Lett. Org. Chem., 2013, 10, 451–456 CrossRef CAS
.
- S. Riyaz, A. Indrasena, A. Naidu and P. Dubey, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2014, 53, 1442–1447 Search PubMed
.
- P. K. Paliwal, S. R. Jetti and S. Jain, Med. Chem. Res., 2013, 22, 2984–2990 CrossRef CAS
.
- Q. Wu, H. Feng, D. D. Guo, M. S. Yi, X. H. Wang, B. Jiang and S. J. Tu, J. Heterocycl. Chem., 2013, 44, 599–602 CrossRef
.
- J. M. Khurana and K. Vij, Synth. Commun., 2013, 43, 2294–2304 CrossRef CAS
.
- C. Han, T. Zhang, A. Zhang, D. Wang, W. Shi and J. Tao, Synthesis, 2014, 46, 1389–1398 CrossRef
.
- G. Mohammadi Ziarani, S. Faramarzi, N. Lashgari and A. Badiei, J. Iran. Chem. Soc., 2014, 11, 701–709 CrossRef CAS
.
- M. Kidwai, A. Jaina, S. Singhb, V. Nemayshb and P. M. Luthrab, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2014, 53, 399–411 Search PubMed
.
- A. Darehkordi, Z. Karimi-Taleghani and O. A. Pouralimardan, J. Iran. Chem. Soc., 2014, 11, 623–629 CrossRef CAS
.
- A. Ganesan, J. Kothandapani, J. B. Nanubolu and S. S. Ganesan, RSC Adv., 2015, 5, 28597–28600 RSC
.
- B. Sadeghi, M. G. Pirbaluti, P. F. Nezhad and R. A. Nezhad, Res. Chem. Intermed., 2015, 41, 4047–4055 CrossRef CAS
.
- R. Kaur, F. Naaz, S. Sharma, S. Mehndiratta, M. K. Gupta, P. M. S. Bedi and K. Nepali, Med. Chem. Res., 2015, 24, 3334–3349 CrossRef CAS
.
- B. Sabour, M. H. Peyrovi and M. Hajimohammadi, Res. Chem. Intermed., 2015, 41, 1343–1350 CrossRef CAS
.
- J. Albadi, A. Mansournezhad and T. Sadeghi, Res. Chem. Intermed., 2015, 41, 8317–8326 CrossRef CAS
.
- A. R. Bhat, A. H. Shalla and R. S. Dongre, Journal of Taibah University for Science, 2016, 10, 9–18 CrossRef
.
- M. A. Bodaghifard, M. Solimannejad, S. Asadbegi and S. Dolatabadifarahani, Res. Chem. Intermed., 2016, 42, 1165–1179 CrossRef CAS
.
- B. Sadeghi, M. Bouslik and M. Shishehbore, J. Iran. Chem. Soc., 2015, 12, 1801–1808 CrossRef CAS
.
- A. Khazaei, A. Ranjbaran, F. Abbasi, M. Khazaei and A. R. Moosavi-Zare, RSC Adv., 2015, 5, 13643–13647 RSC
.
- A. Khazaei, H. A. A. Nik and A. R. Moosavi-Zare, J. Chin. Chem. Soc., 2015, 62, 675–679 CrossRef CAS
.
- M. Keshavarz, J. Iran. Chem. Soc., 2016, 13, 553–561 CrossRef CAS
.
- O. Goli-Jolodar, F. Shirini and M. Seddighi, J. Iran. Chem. Soc., 2015, 13, 457–463 CrossRef
.
- K. Niknam, M. Khataminejad and F. Zeyaei, Tetrahedron Lett., 2016, 57, 361–365 CrossRef CAS
.
- S. H. S. Azzam and M. Pasha, Tetrahedron Lett., 2012, 53, 7056–7059 CrossRef CAS
.
- H. Veisi, A. Maleki, Z. Omrani and S. Lotfi, RSC Adv., 2014, 4, 55313–55317 RSC
.
- X. Yang, L. Yang and L. Wu, Bull. Korean Chem. Soc., 2012, 33, 714–716 CrossRef CAS
.
- G. Mohammadi Ziarani, N. Lashgari, S. Faramarzi and A. Badiei, Acta Chim. Slov., 2014, 61, 574–579 Search PubMed
.
- K. P. Kumar, S. Satyanarayana, P. L. Reddy, G. Narasimhulu, N. Ravirala and B. S. Reddy, Tetrahedron Lett., 2012, 53, 1738–1741 CrossRef
.
- J. Deng, L.-P. Mo, F.-Y. Zhao, Z.-H. Zhang and S.-X. Liu, ACS Comb. Sci., 2012, 14, 335–341 CrossRef CAS PubMed
.
- M. Mirhosseini Moghaddam, A. Bazgir, A. Mohammad Mehdi and R. Ghahremanzadeh, Chin. J. Chem., 2012, 30, 709–714 CrossRef CAS
.
- R.-Y. Guo, P. Wang, G.-D. Wang, L.-P. Mo and Z.-H. Zhang, Tetrahedron, 2013, 69, 2056–2061 CrossRef CAS
.
- H. Naeimi, Z. Rashid, A.-H. Zarnani and R. Ghahremanzadeh, New J. Chem., 2014, 38, 5527–5535 RSC
.
- Q. Cheng, Q. Wang, T. Tan, M. Wang and N. Chen, Chin. J. Chem., 2012, 30, 386–390 CrossRef CAS
.
- L. Y. Zeng, M. F. Lv and C. Cai, Chin. Chem. Lett., 2012, 23, 1347–1351 CrossRef CAS
.
- M. Sharma, P. Borah and P. J. Bhuyan, Synth. Commun., 2015, 45, 1792–1798 CrossRef CAS
.
- S.-S. Jin, H. Wang and H.-Y. Guo, Tetrahedron Lett., 2013, 54, 2353–2356 CrossRef CAS
.
- R. Kazemi-Rad, J. Azizian and H. Kefayati, Tetrahedron Lett., 2014, 55, 6887–6890 CrossRef CAS
.
- G. Harichandran, P. Parameswari, M. Kanagaraj and P. Shanmugam, Tetrahedron Lett., 2015, 56, 150–154 CrossRef CAS
.
- Z. N. Siddiqui, Tetrahedron Lett., 2014, 55, 163–168 CrossRef CAS
.
- P. N. Kalaria, S. P. Satasia and D. K. Raval, New J. Chem., 2014, 38, 1512–1521 RSC
.
- X.-T. Li, A.-D. Zhao, L.-P. Mo and Z.-H. Zhang, RSC Adv., 2014, 4, 51580–51588 RSC
.
- M. M. Heravi, F. Mousavizadeh, N. Ghobadi and M. Tajbakhsh, Tetrahedron Lett., 2014, 55, 1226–1228 CrossRef CAS
.
- J. Li, W. Shi, W. Yang, Z. Kang, M. Zhang and L. Song, RSC Adv., 2014, 4, 29549–29554 RSC
.
- A. Khalafi-Nezhad, M. Nourisefat and F. Panahi, Synthesis, 2014, 46, 2071–2078 CrossRef CAS
.
- A. Alizadeh, R. Ghanbaripour and S. Weng Ng, Helv. Chim. Acta, 2015, 98, 663–667 CrossRef CAS
.
- E. Soleimani, M. Ghanbarian, P. Saei and M. Taran, J. Iran. Chem. Soc., 2015, 12, 2227–2232 CrossRef CAS
.
- M. Mohaqeq, J. Safaei-Ghomi and H. Shahbazi-Alavi, Acta Chim. Slov., 2015, 62, 967–972 CrossRef PubMed
.
- A. Yousefi, R. Yousefi, F. Panahi, S. Sarikhani, A. R. Zolghadr, A. Bahaoddini and A. Khalafi-Nezhad, Int. J. Biol. Macromol., 2015, 78, 46–55 CrossRef CAS PubMed
.
- A. Khalafi-Nezhad and F. Panahi, Synthesis, 2011, 984–992 CrossRef CAS
.
- J. Quiroga, S. Portillo, A. Pérez, J. Gálvez, R. Abonia and B. Insuasty, Tetrahedron Lett., 2011, 52, 2664–2666 CrossRef CAS
.
- S. A. Shirvan, R. Ghahremanzadeh, M. M. Moghaddam, A. Bazgir, A. H. Zarnani and M. M. Akhondi, J. Heterocycl. Chem., 2012, 49, 951–954 CrossRef CAS
.
- R. Ghahremanzadeh, Z. Rashid, A.-H. Zarnani and H. Naeimi, Dalton Trans., 2014, 43, 15791–15797 RSC
.
- A. Kamal, K. S. Babu, M. V. Vardhan, S. A. Hussaini, R. Mahesh, S. P. Shaik and A. Alarifi, Bioorg. Med. Chem. Lett., 2015, 25, 2199–2202 CrossRef CAS PubMed
.
- K. Balamurugan, S. Perumal and J. C. Menéndez, Tetrahedron, 2011, 67, 3201–3208 CrossRef CAS
.
- G.-P. Lu and C. Cai, J. Chem. Res., 2011, 35, 547–551 CrossRef CAS
.
- V. O. Iaroshenko, S. Dudkin, V. Y. Sosnovskikh, A. Villinger and P. Langer, Synthesis, 2013, 45, 971–977 CrossRef CAS
.
- A. Rahmati and Z. Khalesi, Tetrahedron, 2012, 68, 8472–8479 CrossRef CAS
.
- N. Azizi, A. Mobinikhaledi, A. K. Amiri and H. Ghafuri, Res. Chem. Intermed., 2012, 38, 2271–2275 CrossRef CAS
.
- A. Khalafi-Nezhad, S. Sarikhani, E. S. Shahidzadeh and F. Panahi, Green Chem., 2012, 14, 2876–2884 RSC
.
- M. H. Mosslemin, E. Zarenezhad, N. Shams, M. N. S. Rad, H. Anarakiardakani and R. Fayazipoor, J. Chem. Res., 2014, 38, 169–171 CrossRef CAS
.
- R. Ghahremanzadeh, M. M. Moghaddam, A. Bazgir and M. M. Akhondi, Chin. J. Chem., 2012, 30, 321–326 CrossRef CAS
.
- A. Khalafi-Nezhad, M. Divar and F. Panahi, Tetrahedron Lett., 2013, 54, 220–222 CrossRef CAS
.
- M. Nourisefat, F. Panahi and A. Khalafi-Nezhad, Org. Biomol. Chem., 2014, 12, 9419–9426 CAS
.
- A. Khalafi-Nezhad, F. Panahi and B. Golesorkhi, Helv. Chim. Acta, 2013, 96, 1155–1162 CrossRef CAS
.
- N. O. Mahmoodi, S. Ramzanpour and F. Ghanbari Pirbasti, Arch. Pharm., 2015, 348, 275–282 CrossRef CAS PubMed
.
- F. Panahi, R. Yousefi, M. H. Mehraban and A. Khalafi-Nezhad, Carbohydr. Res., 2013, 380, 81–91 CrossRef CAS PubMed
.
- R. Memarzadeh, H.-B. Noh, S. Javadpour, F. Panahi, A. Feizpour and Y.-B. Shim, Bull. Korean Chem. Soc., 2013, 34, 2291–2296 CrossRef
.
- A. Khalafi-Nezhad and S. Mohammadi, ACS Comb. Sci., 2013, 15, 512–518 CrossRef CAS PubMed
.
- S. Abdolmohammadi, S. Balalaie, M. Barari and F. Rominger, Comb. Chem. High Throughput Screening, 2013, 16, 150–159 CAS
.
- A. R. Bhat and R. S. Dongre, J. Taiwan Inst. Chem. Eng., 2015, 56, 191–195 CrossRef CAS
.
- G. Mohammadi Ziarani, P. Gholamzadeh, A. Badiei, S. Asadi and A. Abolhasani Soorki, J. Chil. Chem. Soc., 2015, 60, 2975–2978 CrossRef
.
- R. Yousefi, M.-M. Alavian-Mehr, F. Mokhtari, F. Panahi, M. H. Mehraban and A. Khalafi-Nezhad, J. Enzyme Inhib. Med. Chem., 2013, 28, 1228–1235 CrossRef CAS PubMed
.
- Z. Toobaei, R. Yousefi, F. Panahi, S. Shahidpour, M. Nourisefat, M. M. Doroodmand and A. Khalafi-Nezhad, Carbohydr. Res., 2015, 411, 22–32 CrossRef CAS PubMed
.
- A. Rahmati and M. Eskandari-Vashareh, J. Chem. Sci., 2014, 126, 169–176 CrossRef CAS
.
- N. Kozlov and A. Tereshko, Russ. J. Org. Chem., 2014, 50, 1006–1011 CrossRef CAS
.
- M. Yaqub, N. Arif, R. Perveen, J. Batool, M. T. Riaz and M. Yaseen, Asian J. Chem., 2015, 27, 1013 CrossRef CAS
.
- J. Khalafy, M. Rimaz, L. Panahi and H. Rabiei, Bull. Korean Chem. Soc., 2011, 42, 2428–2432 CrossRef
.
- D. Prajapati, D. Bhuyan, M. Gohain and W. Hu, Mol. Diversity, 2011, 15, 257–261 CrossRef CAS PubMed
.
- G. Mohammadi Ziarani, S. Faramarzi, S. Asadi and M. Amanlou, Iran. J. Pharm. Res., 2015, 14, 1105–1114 Search PubMed
.
- M. Nasr-Esfahani and M. Taei, RSC Adv., 2015, 5, 44978–44989 RSC
.
- A. Mobinikhaledi, T. Mosleh and N. Foroughifar, Res. Chem. Intermed., 2015, 41, 2985–2990 CrossRef CAS
.
- A. K. Arya and M. Kumar, Green Chem., 2011, 13, 1332–1338 RSC
.
- R. Gupta, A. Jain, R. Joshi and M. Jain, Bull. Korean Chem. Soc., 2011, 32, 899–904 CrossRef CAS
.
- G. Meshram, P. Wagh, S. Deshpande and V. Amratlal, Lett. Org. Chem., 2013, 10, 445–450 CrossRef CAS
.
- M. N. Elinson, V. M. Merkulova, A. I. Ilovaisky, F. Barba and B. Batanero, Electrochim. Acta, 2011, 56, 8219–8223 CrossRef CAS
.
- A. Rahmati and K. Vakili, Helv. Chim. Acta, 2012, 95, 1126–1135 CrossRef CAS
.
- K. Eskandari, B. Karami, M. Farahi and V. Mouzari, Tetrahedron Lett., 2016, 57, 487–491 CrossRef CAS
.
- S. J. Kalita, H. Mecadon and D. C. Deka, Tetrahedron Lett., 2015, 56, 731–734 CrossRef
.
- Y. Dommaraju, S. Borthakur, N. Rajesh and D. Prajapati, RSC Adv., 2015, 5, 24327–24335 RSC
.
- A. Andina and A. Andin, Russ. J. Org. Chem., 2015, 51, 1043–1045 CrossRef CAS
.
- M. Li, B. Zhang and Y. Gu, Green Chem., 2012, 14, 2421–2428 RSC
.
- R. Sangsuwan, S. Sangher, T. Aree, C. Mahidol, S. Ruchirawat and P. Kittakoop, RSC Adv., 2014, 4, 13708–13718 RSC
.
- M. N. Elinson, R. F. Nasybullin, O. O. Sokolova, T. A. Zaimovskaya and M. P. Egorov, Monatsh. Chem., 2015, 146, 1689–1694 CrossRef CAS
.
- Y. Dommaraju and D. Prajapati, Mol. Diversity, 2015, 19, 173–187 CrossRef CAS PubMed
.
- T. Demirci, M. Arslan, Ç. Bilen, D. Demir, N. Gençer and O. Arslan, J. Enzyme Inhib. Med. Chem., 2014, 29, 132–136 CrossRef CAS PubMed
.
- V. Padmavathi, G. D. Reddy, B. C. Venkatesh and A. Padmaja, Arch. Pharm., 2011, 344, 165–169 CrossRef CAS PubMed
.
- A. Habibi, Y. Valizadeh, A. Alizadeh, H. A. Rudbari and V. M. Nardo, J. Sulfur Chem., 2014, 35, 362–372 CrossRef CAS
.
- M. Faryabi and E. Sheikhhosseini, J. Iran. Chem. Soc., 2015, 12, 427–432 CrossRef CAS
.
- V. Reddy Mudumala, R. Rani Chinthaparthi and T. Jeong Yeon, Tetrahedron, 2014, 70, 3762–3769 CrossRef CAS
.
- A. Biabangard and H. R. Shaterian, J. Iran. Chem. Soc., 2015, 12, 1529–1534 CrossRef CAS
.
- A. Barakat, A. M. Al-Majid, H. J. Al-Najjar, Y. N. Mabkhot, S. Javaid, S. Yousuf and M. I. Choudhary, Eur. J. Med. Chem., 2014, 84, 146–154 CrossRef CAS PubMed
.
- M. Bihani, P. P. Bora, A. K. Verma, R. Baruah, H. P. D. Boruah and G. Bez, Bioorg. Med. Chem. Lett., 2015, 25, 5732–5736 CrossRef CAS PubMed
.
- I. Yavari, A. Aminkhani and S. Arab-Salmanabadi, Monatsh. Chem., 2012, 143, 1195–1198 CrossRef CAS
.
- H. Hou, Y. Zhang and C.-G. Yan, Chem. Commun., 2012, 4492–4494 RSC
.
- V. A. Peshkov, A. A. Peshkov, O. P. Pereshivko, K. Van Hecke, L. L. Zamigaylo, E. V. Van der Eycken and N. Y. Gorobets, ACS Comb. Sci., 2014, 16, 535–542 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |