A facile method to fabricate carbon nanostructures via the self-assembly of polyacrylonitrile/poly(methyl methacrylate-b-polyacrylonitrile) AB/B′ type block copolymer/homopolymer blends
A. Palanisamya,
N. V. Salima,
B. L. Foxb,
P. Jyotishkumarc,
T. Pradeepd and
N. Hameed*abd
aInstitute for Frontier Materials, Deakin University, Geelong, Australia
bFactory of the Future, Swinburne University of Technology, Hawthorn, Australia. E-mail: nisharhameed@swin.edu.au
cDepartment of Polymer Science and Rubber Technology, Cochin University of Science and Technology, Cochin, India
dDST Unit of Nanoscience (DST UNS) and Thematic Unit of Excellence (TUE), Department of Chemistry, Indian Institute of Technology Madras, Chennai, India
First published on 1st June 2016
Abstract
The self-assembly and high temperature behavior of AB/B′ type block copolymer/homopolymer blends containing polyacrylonitrile (PAN) polymers were studied for the first time. Here, microphase separated nanostructures were formed in the poly(methyl methacrylate-b-polyacrylonitrile) (PMMAN) block copolymer and their blends with homopolymer PAN at various blend ratios. Additionally, these nanostructures were transformed into porous carbon nanostructures by sacrificing PMMA blocks via pyrolysis. Spherical and worm like morphologies were observed in both TEM and AFM images at different compositions. The thermal and phase behavior examinations showed good compatibility between the blend components in all studied compositions. The PAN homopolymer (B′) with a comparatively higher molecular weight than the corresponding block (B) of the block copolymer is expected to exhibit ‘dry brush’ behavior in this AB/B′ type system. This study provides a basic understanding of the miscibility and phase separation in the PMMAN/PAN system, which is important in the nanostructure formation of bulk PAN based materials with the help of block copolymers to develop advanced functional materials.
Introduction
Polyacrylonitrile (PAN) is one of the important industrial polymers used as a precursor for the production of carbon fibers. In recent years, carbon fibers have found application in a wide range of industries such as robotics, marine, aerospace, electronics, medical and construction markets. In order to improve their inherent properties and to diversify their applications, the PAN precursor is either copolymerized with other monomers or blended with components of interest. The phase separation and morphology of polyacrylonitrile (PAN) based block copolymers are of great interest in recent years due to the commercial potential of PAN in various niche markets.1,2 Various processes were used to synthesize PAN block copolymers with different architectures in order to achieve complex nanostructured phases by self-assembly.3,4 As catalytic and anionic polymerization process were unable to produce linear polymers,5 controlled radical polymerization methods such as reversible addition fragmentation chain transfer (RAFT), nitroxide-mediated radical polymerization (NMP) and atom transfer radical polymerization (ATRP) were used to synthesize block copolymers of varied compositions.6 However, the synthesis of PAN block copolymers for achieving different morphologies was a challenge due to poor solubility of PAN in the acrylonitrile monomer and in common organic solvents, incompatibility among the co-blocks and difficulty with controlling the initiation efficiency of the macroinitiator.7 In addition to available synthetic techniques, blending of PAN containing block copolymers with homopolymers may provide opportunity to reach at various equilibrium morphologies. In blends, the homopolymer can swell the respective block copolymer domains and can unravel new nanostructures depending on the composition. Additionally, the blending technique may eliminate the requirement for precise control of chain length during polymerization to reach at predetermined morphology. Especially in PAN polymer systems, nano-structuration of PAN domains can be achieved via simple blending to enhance the bulk material properties.Block copolymers of PAN with various co-blocks such as poly(ethylene oxide),8 polystyrene,7 methyl acrylate, itaconic acid9 and poly(n-butyl acrylate)10 were synthesized via various polymerization techniques. Microphase separated morphologies such as spheres and cylinders of PAN domains also were identified. In addition to diblock copolymers, PAN containing triblock copolymers of various composition and architectures were found in literature. For instance, phase separation in ABA triblock copolymer of polyacrylonitrile and poly(n-butyl acrylate) resulted in spherical morphology of PAN domains in butyl acrylate matrix.11 Besides phase separation, the good membrane forming ability of PAN was exploited to prepare dehydration membranes by copolymerizing PAN with hydrophilic polymers such as poly(ethylene oxide).12 PAN containing block copolymer membranes was also investigated for separating organic solvent mixtures, where, block copolymer composition plays important role in morphology and phase inversion.13 In addition to membrane development, the unique property of PAN to form carbon based materials upon pyrolysis has drawn great research interest.14 Self-assembled lamellar thin films of poly(n-butyl acrylate)-b-polyacrylonitrile was carbonized to prepare ordered nanostructured carbon.15 Carbon based material such as carbon nanoparticles were also prepared from self-assembled micelles of poly(acrylic acid)-b-polyacrylonitrile copolymer in aqueous solution.16 These simple spherical and lamellar carbon nanostructures can be scaled up to more complex nanostructures via tailored synthesis of PAN containing block copolymers coupled with blending strategy.
Polyacrylonitrile has been blended with various functional materials such as Nafion,17 cellulose18 and carbon nanotubes19 to improve native properties of these materials. In the same vein, PAN has also been used to improve the properties of electro spun membranes of poly(vinylidene fluoride-co-hexafluoropropylene) block copolymer via blending.20 However, blends of PAN homopolymer with block copolymers and their self-assembled morphology were not studied in detail till date. Even though nanostructured carbon has been prepared from PAN containing block copolymers,15,16 blending of PAN homopolymers with block copolymers may be a straight forward route to develop more complex morphologies by avoiding synthesis of tailored polymers. Specifically, simple AB/B′ type polymer blend system is investigated in this study. The B and B′ blocks represents the PAN blocks of block copolymer and homopolymer respectively. The A block may be of any polymer that can modify or improve the properties of PAN. Depending on the molecular weight ratio of the B′ to B, these systems exhibit three types of behavior, namely, (a) wet brush (molecular weight of B′ < B), (b) dry brush (B′ > B) and (c) macrophase separation (B′ ≫ B).21 Here the blends show all these behaviors depending on the concentration of homopolymer. As the molecular weight (concentration) of B′ increases, the solubility of B′ in B decreases, this changes wet brush behavior to dry brush and finally leading to complete macrophase separation. In general, the homopolymer swells the corresponding block copolymer domain to varying degree depending on molecular weight and bring new self-assembled morphologies that can be transferred into nanostructured carbon materials. Developing a straightforward and industrially feasible method of producing carbon nanostructures of various morphologies via simple blending technique there by avoiding complex synthetic procedures was the main driving force behind this research. To our knowledge, self-assembled morphology in AB/B′ type blends containing PAN is studied here for the first time. Additionally, this study provides new knowledge on, ways to develop porous carbon nanostructures from block copolymer blends containing sacrificial block (PMMA) and determining blend compositions that lead to equilibrium morphologies without macrophase separation in systems with high molecular weight PAN. This study may also provide basic understanding of miscibility and morphology control in PAN containing AB/B′ type blends and provide new direction towards nanostructuring PAN polymers.
In this study, blends of poly(methyl methacrylate-b-polyacrylonitrile) and PAN were prepared in various compositions and studied their self-assembled morphology. The possible mechanism for morphology transformation was also proposed. The self-assembled morphology was transferred to carbon nanostructures via pyrolysis and the morphology was studied using electron microscopy. This study may create a paradigm for future studies in developing highly ordered nanostructured carbon materials such as mesoporous carbon, porous membranes and porous carbon nanofibers via simple blending of PAN homopolymers with block copolymers. The porous morphology of these carbon nanostructures is particularly suitable for developing electrodes with high specific capacitance for energy storage and porous carbon nanostructures with high surface area may find application in catalysis as well.
Materials and methods
Materials and preparation of blends
The poly(methyl methacrylate-b-polyacrylonitrile) (PMMAN) block copolymer was obtained from Polymer Source, Inc. The PMMAN was with an average Mn (PMMA) = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mn (PAN) = 8700 and Mw/Mn = 1.25. Polyacrylonitrile with average Mw = 150
000, Mn (PAN) = 8700 and Mw/Mn = 1.25. Polyacrylonitrile with average Mw = 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was purchased from Sigma-Aldrich Co (Chemical structures of polymers used in this study shown in Fig. 1a). Both the homopolymer and block copolymer were individually dissolved in dimethylformamide (DMF) to get 1% (w/v) solution. The blends were prepared via mixing the polymer solutions at different weight ratios. The mixtures were poured into an aluminum pan and solvent was allowed to evaporate slowly at room temperature. Before measurements, the blends were dried in a vacuum oven followed by annealing at 80 °C for 72 hours.
000 was purchased from Sigma-Aldrich Co (Chemical structures of polymers used in this study shown in Fig. 1a). Both the homopolymer and block copolymer were individually dissolved in dimethylformamide (DMF) to get 1% (w/v) solution. The blends were prepared via mixing the polymer solutions at different weight ratios. The mixtures were poured into an aluminum pan and solvent was allowed to evaporate slowly at room temperature. Before measurements, the blends were dried in a vacuum oven followed by annealing at 80 °C for 72 hours.
 | ||
| Fig. 1 (a) Chemical structures of PMMAN block copolymer and PAN homopolymer, (b) FTIR spectra of various PMMAN/PAN blends at room temperature. | ||
Differential scanning calorimetry (DSC)
Calorimetric measurements were made on a TA Q200 differential scanning calorimeter instrument. Around 8 mg of sample was used and all measurements were performed in nitrogen atmosphere. The samples were held at 30 °C for 3 min and heated to 400 °C at the rate of 20 °C min−1.Thermogravimetric analysis (TGA)
Thermogravimetric measurements were made on a TA Q50 instrument. Around 8 mg of sample was used and all measurements were performed in nitrogen atmosphere. The samples were heated to 800 °C at the rate of 20 °C min−1.Transmission electron microscopy (TEM)
The DMF solution of polymer mixture was dropped on a carbon coated copper grid and a thin film of the sample was allowed to dry in room temperature for at least 24 hours. The grids were then annealed at 80 °C for 72 hours before imaging. JEOL JEM-2100 electron microscope operating at an acceleration voltage of 100 kV was used to study the morphology of blends.Fourier transform infrared (FTIR) spectroscopy
The thin films of annealed samples were used for FTIR measurements. FTIR analysis of the samples were performed with a Bruker Vertex-70 FTIR spectrometer with a Pike ATR high-pressure clamp. An average of 32 scans in the standard wavenumber range 600–4000 cm−1 at a resolution of 4 cm−1 was recorded.Atomic force microscopy (AFM)
The DMF solution of polymer mixture was spin coated on to a clean silicon surface at 3000 rpm and air dried for 24 hours at room temperature. The spin coated samples were then annealed at 80 °C for 72 hours before imaging. The surface morphology was then studied in Bruker Multimode™ 8 SPM instrument using a silicon cantilever of spring constant 42 N m−1 in tapping mode. The height images were recorded and analyzed using NanoScope Analysis software.Scanning electron microscopy (SEM)
The samples spin coated on a silicon wafer for AFM analysis was used for SEM observation after carbonization at 700 °C in inert atmosphere. The samples were coated with a thin layer (3 nm) of gold and imaged at 5 kV with a working distance of 4.5 mm. A Zeiss Supra 55 VP field emission gun scanning electron microscope was used for imaging.Results and discussion
The interaction between the PMMAN block copolymer and PAN homopolymer was studied by FTIR spectroscopy. Fig. 1b shows the FTIR spectra of PMMAN block copolymer, PAN homopolymer and PMMAN/PAN blends at various compositions. In all cases, the distinct absorption bands between 2827–3050 cm−1 and 1149–1475 cm−1 were assigned to C–H stretching and various C–H bending vibration modes in CH2, CH groups respectively. The band at 2245 cm−1 resulted from C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching of the nitrile groups (CN) of PAN. The band around 1664 cm−1 may be due to N–H stretching in acrylamide or pyridone type structures formed during PAN polymerization reaction.22,23 Even though PAN is one of the blocks in block copolymer, this band is more pronounced in PAN homopolymer and in PMMAN/PAN blends. The C
N stretching of the nitrile groups (CN) of PAN. The band around 1664 cm−1 may be due to N–H stretching in acrylamide or pyridone type structures formed during PAN polymerization reaction.22,23 Even though PAN is one of the blocks in block copolymer, this band is more pronounced in PAN homopolymer and in PMMAN/PAN blends. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching from PMMA block of the block copolymer was observed at 1726 cm−1. In blends, the intensity of nitrile group stretching band at 2245 cm−1 can be observed to increase with increasing the PAN content. The carbonyl stretching band at 1726 cm−1 follows the same trend with increasing block copolymer content.
O stretching from PMMA block of the block copolymer was observed at 1726 cm−1. In blends, the intensity of nitrile group stretching band at 2245 cm−1 can be observed to increase with increasing the PAN content. The carbonyl stretching band at 1726 cm−1 follows the same trend with increasing block copolymer content.
There was no significant shift in absorption bands upon changing the composition. However, slight shift has been observed in N–H stretching band at 1664 cm−1 (Fig. 2). Upon the addition of PMMAN block copolymer, the graphs appear to shift towards higher wavenumber region. This may be due to possible interaction among acrylamide or pyridone groups present in PAN homopolymer.
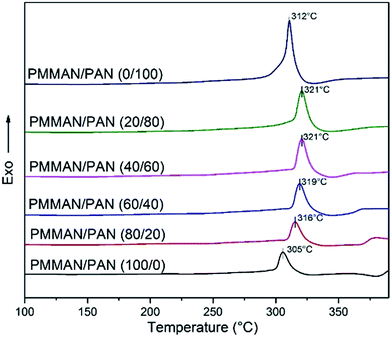
The thermal behavior of the blends was studied using DSC and TGA analysis. Fig. 3 represents the DSC analysis of PAN homopolymer, PMMAN block copolymer and their blends at various compositions. Table 1 shows the DSC parameters of the blends. The study of thermal stabilization of PAN based polymers is important in obtaining novel carbon fibre precursors for various applications.2 Here, the change in temperature of cyclization reaction in PAN was studied at various PMMA contents by changing the block copolymer content. The exothermic peaks in first heating cycle were shown in the Fig. 3. In terms of cyclization temperature (Tc), for pure homopolymer (PAN) and block copolymer (PMMAN), the Tc was 312 °C and 305 °C respectively. As expected, the Tc for the block copolymer was lower than the pure PAN. The heat flow and the initial and peak temperatures are low in block copolymer due to presence of PMMA blocks.24
| Composition (PMMAN/PAN) | Initial exothermic temp. (°C) | End exothermic temp. (°C) | Cyclization temp, Tc (°C) | Difference between initial and end exothermic temp. (°C) | Heat energy ΔHc (J g−1) |
|---|---|---|---|---|---|
| 0/100 | 235.2 | 330.4 | 312.1 | 95.2 | 349.3 |
| 20/80 | 233.8 | 344.8 | 321.8 | 111.0 | 296.8 |
| 40/60 | 244.6 | 345.2 | 321.7 | 100.6 | 271.7 |
| 60/40 | 247.1 | 345.9 | 319.8 | 98.8 | 240.8 |
| 80/20 | 248.5 | 340.5 | 316.0 | 92.0 | 178.7 |
| 100/0 | 241.7 | 330.4 | 305.7 | 88.7 | 140.7 |
According to the data in Table 1, the blend at 80/20 ratio of PMMAN/PAN showed an increase in Tc (316 °C) compared to the pure block copolymer (305 °C). Due to comparatively higher molecular weight of PAN homopolymer (Mw = 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) than the PAN block (Mn = 8700) in the copolymer, the total PAN content or molar ratio of PAN to PMMA must be higher in all compositions, leading to increased Tc. Upon further increase in PAN content, PMMAN/PAN = 20/80, Tc increased to 321 °C.
000) than the PAN block (Mn = 8700) in the copolymer, the total PAN content or molar ratio of PAN to PMMA must be higher in all compositions, leading to increased Tc. Upon further increase in PAN content, PMMAN/PAN = 20/80, Tc increased to 321 °C.
In addition to the decrease in cyclization temperature upon increasing total PMMA content, the temperature difference between initial and end exothermic peak decreased. This implies that the cyclization reaction takes place within a comparatively narrow window of temperature at higher PMMA content.25,26 The energy required for cyclization of nitrile groups decreased at higher PMMA contents can be evidenced from the measure of heat of cyclization (ΔHc) (Table 1). In general, the change in thermal properties with change in composition indicates the complete miscibility of PAN homopolymer in spite of its higher molecular weight.
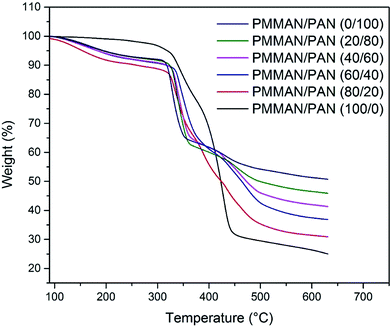
Fig. 4 shows the TGA curves of PMMAN/PAN bends at various compositions. The weight change can be expressed in four succinct steps. The first step occurred at low temperature region (150–300 °C), around 8% weight loss may be due to residual moisture and decomposition of low molecular weight components.27,28 In pure block copolymer samples, only 3% weight loss was observed in above temperature region. The second weight loss of ∼27% started around 300 °C and can be attributed to nitrile oligomerization and loss of other volatile gaseous products.29 Around 16–22% weight loss between 365 and 490 °C was due to thermal degradation of polymers.30 In final stage, 7–10% of weight loss was observed due to pyrolytic degradation of PAN and yielded a residual mass of 25–50% at 650 °C depending on the total PAN content. A gradual increase in residual mass was observed upon increasing PAN content (Fig. 4). From the thermograms, it is evident that the samples with higher PAN content have higher thermal stability compared to pure PMMAN.
The self-assembled morphology of the blends was studied using TEM, SEM and AFM techniques. Fig. 5 and 6 are TEM and AFM images of blends at various compositions. In pure PMMAN bock copolymer, the shorter PAN blocks formed spherical micelles with longer poly(methyl methacrylate) blocks forming the continuous phase. In TEM and AFM images (Fig. 5a and d), the phase separated PAN block can be observed as spherical dots in a continuous PMMA phase. Even though the samples were not stained for TEM observation, good contrast between the phase separated domains was observed, which may be due to the electron density difference among them. The self-assembled morphology of block copolymers can be tuned by adding a homopolymer that is similar to one of the blocks of the block copolymer. In these AB/B′ type systems without specific interaction between blend components, the molecular weight of homopolymer plays a critical role in dictating the self-assembly.31 In general, the polymer brush structure changes from ‘wet brush’ to ‘dry brush’ regime upon increasing the molecular weight of homopolymer low to high relative to a block of the block copolymer.32 When the molecular weight of homopolymer is comparable or smaller than corresponding block of the block copolymer, homopolymer molecules can penetrate and exhibit wet brush behavior. If the molecular weight ratio is higher, then dry brush behavior (simple tether of polymer chains at interface) is observed.33 Here, the PAN homopolymer may be in the dry brush regime. The low molecular weight PAN chains in the block copolymer may be tethered at the interface of higher molecular weight PAN homopolymer domains at the cost of conformational entropy loss. Hence, at PMMAN/PAN = 80/20, phase inversion was expected because the total PAN content was higher compared to PMMA. The PMMA blocks formed worm like structures in addition to spherical micelles in the continuous phase of PAN (Fig. 5b and e).
 | ||
| Fig. 5 TEM images of PMMAN/PAN blends at ratios (a) 100/0, (b) 80/20, (c) 60/40 and their corresponding AFM images (d)–(f). | ||
 | ||
| Fig. 6 TEM images of PMMAN/PAN blends at ratios (a) 40/60, (b) 20/80, (c) 0/100 and their corresponding AFM images (d)–(f). | ||
On further increasing the concentration of PAN, at PMMAN/PAN = 60/40 and 40/60, the PMMA blocks completely transformed into spherical structures as observed in Fig. 5c and f and 6a and b. In this system, the difference in molecular weight between the PAN block of the block copolymer and the PAN homopolymer was large. Hence, the intermediate morphologies such as lamellar structures couldn't be observed due to morphology transition occurred in a narrow window of composition. However, macrophase separation of PAN homopolymer was not observed despite its higher molecular weight. At higher PAN concentration, PMMAN/PAN = 20/80 and 0/100, the PAN homopolymer exhibited rough surface can be evidenced from TEM and AFM images (Fig. 6). However, PMMA domains can be observed as spherical dots in TEM image at 20/80 ratio.
Fig. 7 shows the schematic representation of morphology transformation and phase inversion upon changing the blend composition. The possible mechanism for morphology change and phase inversion is explained in this section. It is well known that in asymmetric block copolymers, relatively smaller block self-assemble into spherical micelles in a continuous matrix of the larger block.34 Here, in self-assembled PMMAN block copolymer, the spherical domains of PAN blocks are represented as red spheres in a blue PMMA matrix (Fig. 7a). In AB/B type systems, the ‘B’ homopolymer with equal or lower molecular weight than the corresponding block of the block copolymer generally exhibit ‘wet brush’ behavior.35 Here, the PAN homopolymer with comparatively higher molecular weight than the PAN block of block copolymer showed a ‘dry brush’ behavior. In Fig. 7b, the spherical and worm like domains of PMMA blocks are shown in continuous phase of PAN. Due to higher molecular weight PAN matrix, PMMA domains are expected to be stabilized by low molecular weight PAN blocks at the interface. The complete macrophase separation of PAN and PMMAN was not observed at any compositions. Hence, at this PAN molecular weight ratio, neither ‘wet brush’ nor ‘macrophase separation’ was observed. The molecular weight of PAN homopolymer used in this study was right enough to obtain morphology change without macrophase separation between PMMAN and PAN. The shorter PAN chains at the interface of PMMA domains and PAN matrix was expected to stabilize the phase separated nanostructures there by avoiding macrophase separation. In blends of, either higher molecular weight PAN homopolymer or block copolymer with lower PAN content may lead to macrophase separation due to destabilization of phase separated domains. Hence, in polymer blends with PAN molecular weight ratios less than or equal to the one used in this study may be used control the morphology without macrophase separation. Due to higher conformational entropy penalty in ‘dry brush’ systems, change in domain size and morphology transformation cannot be observed to greater extent upon changing the composition.35 Additionally, due to higher total weight fraction of PAN compared to PMMA, the phase inversion was observed at ratio PMMAN/PAN = 80/20 and higher. The worm like micelles are the intermediate morphology observed coexisting with spherical PMMA domains. Further at higher PAN content, PMMAN/PAN = 60/40 (Fig. 7c), the worm like micelles disappeared and spherical micelles were the only morphology observed. Since the homopolymers doesn't dissolve in corresponding block of the block copolymer in ‘dry brush’ systems, one cannot expect highly ordered morphologies. However, careful selection of blend components with molecular weight ratios of PAN within the ‘wet brush’ regime may result in novel morphologies in PAN containing block copolymer systems.
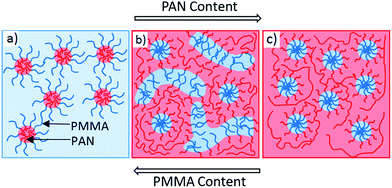 | ||
| Fig. 7 Schematic representation of morphology transformation upon changing the blend compositions PMMAN/PAN (a) 100/0 (b) 80/20 and (c) 60/40. | ||
The PAN containing block copolymers are well known for development of nanostructured porous carbon after annealing by various self assembly techniques.11,36 Here, the morphology of these blends were further explored using SEM after thermal treatment at 700 °C in inert atmosphere. In pure block copolymer samples, porous carbon nanostructures can be observed after sacrificing PMMA blocks during carbonization (Fig. 8a). The weight loss during carbonization leads to significant volume reduction and change in the film properties.37 Eventhough the PAN domains in the pure block copolymer appear to be separated by few nanometres (Fig. 5a), interconnected porous structures are observed after removing PMMA blocks. At PMMAN/PAN = 50/50, the surface of the carbonized film appears to contain small pores after removal of PMMA domains (Fig. 8b). The morphology of carbonized films from samples with higher PMMA (PMMAN/PAN = 100/0) and PAN (PMMAN/PAN = 50/50) content differ by the density and arrangement of pores. The pure PAN homopolymer resulted in formation of bigger carbon particles after carbonization can be seen in Fig. 8c.
 | ||
| Fig. 8 SEM image of carbonized samples of PMMAN/PAN blends at ratios (a) 100/0, (b) 50/50 (inset scale bar, 200 nm), (c) 0/100. | ||
This study examines the morphology evolution in PAN containing blends, hence these morphologies can be transferred into carbon nanostructures. This hypothesis has been tested here with porous nanostructures have been obtained as expected and possible mechanism for morphology transformation have also been provided. In principle, the pore size and morphology depends on multiple factors such as sacrificial block length, composition of the block copolymer and molecular weight of the homopolymer. By careful selection of the blend components, the desired highly ordered burnt morphologies can be achieved. The average pore size should be in the range of 10–50 nm and these porous nanostructures with high surface area can be used in catalysis and in development of electrodes.
Conclusions
We studied the phase behavior and self-assembled morphology of PMMAN/PAN blends at various compositions. Our results reveal that the microphase separation in PAN containing block copolymers can be controlled via adding PAN homopolymer of comparatively higher molecular weight. In pure PMMAN block copolymer, spherical domains of PAN were observed in PMMA matrix and followed by phase inversion at higher PAN contents. Upon increasing the ratio of PMMAN/PAN from 80/20 to 20/80, worm like PMMA domains were transformed into spherical domains. Furthermore, PMMA domains were uniformly distributed in the PAN matrix irrespective of the large molecular weight difference between PAN blocks, which confirms the ‘dry brush’ behavior in this AB/B′ system. The basic understanding and knowledge of miscibility and phase separation derived from this system can be applied to similar AB/B′ systems with various co-blocks. The porous carbon nanostructures derived from pyrolysis of the self-assembled blends may be first step towards nanostructuration of PAN based materials via simple blending technique. Hence, careful selection of molecular weight of blend components within the ‘wet’ or ‘dry brush’ regime may help in capturing many intermediate morphologies that can be transformed into carbon nanostructures of interest. Also, this simple blending strategy would help in identifying novel precursors for preparation of nanostructured carbon materials instead of following complex polymer synthetic protocols. We believe this preliminary study would create interest towards using this blending strategy wherever possible in the process of nanostructuring PAN based materials.Acknowledgements
The authors would like to thank VESKI for the Victoria Fellowship (N. H.), Deakin University IFM Impact grant and Deakin advanced electron microscopy facility.Notes and references
- A. Asatekin, E. A. Olivetti and A. M. Mayes, Fouling resistant, high flux nanofiltration membranes from polyacrylonitrile-graft-poly(ethylene oxide), J. Membr. Sci., 2009, 332(1–2), 6–12 CrossRef CAS.
- S. K. Nataraj, K. S. Yang and T. M. Aminabhavi, Polyacrylonitrile-based nanofibers—A state-of-the-art review, Prog. Polym. Sci., 2012, 37(3), 487–513 CrossRef CAS.
- M. Baumann, et al., Synthesis of polystyrene-block-poly(styrene-co-acrylonitrile) block copolymers and thermoanalytical studies of nitroxide-terminated poly(styrene-co-acrylonitrile) copolymers, Macromol. Mater. Eng., 2000, 280–281(1), 1–6 CrossRef.
- N. V. Tsarevsky, et al., Synthesis of Styrene–Acrylonitrile Copolymers and Related Block Copolymers by Atom Transfer Radical Polymerization, Macromolecules, 2002, 35(16), 6142–6148 CrossRef CAS.
- F. Schaper, S. R. Foley and R. F. Jordan, Acrylonitrile Polymerization by Cy3PCuMe and (Bipy)2FeEt2, J. Am. Chem. Soc., 2004, 126(7), 2114–2124 CrossRef CAS PubMed.
- C. Tang, T. Kowalewski and K. Matyjaszewski, Preparation of Polyacrylonitrile-block-poly(n-butyl acrylate) Copolymers Using Atom Transfer Radical Polymerization and Nitroxide Mediated Polymerization Processes, Macromolecules, 2003, 36(5), 1465–1473 CrossRef CAS.
- M. Lazzari, et al., Synthesis of Polyacrylonitrile-block-Polystyrene Copolymers by Atom Transfer Radical Polymerization, Macromol. Chem. Phys., 2005, 206(14), 1382–1388 CrossRef CAS.
- K. Sui and L. Gu, Preparation and characterization of amphiphilic block copolymer of polyacrylonitrile-block-poly(ethylene oxide), J. Appl. Polym. Sci., 2003, 89(7), 1753–1759 CrossRef CAS.
- A. V. Shlyahtin, et al., Synthesis of polyacrylonitrile copolymers as potential carbon fibre precursors in CO2, Green Chem., 2014, 16(3), 1344–1350 RSC.
- B. Dufour, et al., Polar Three-Arm Star Block Copolymer Thermoplastic Elastomers Based on Polyacrylonitrile, Macromolecules, 2008, 41(7), 2451–2458 CrossRef CAS.
- T. Kowalewski, N. V. Tsarevsky and K. Matyjaszewski, Nanostructured Carbon Arrays From Block Copolymers of Polyacrylonitrile, J. Am. Chem. Soc., 2002, 124(36), 10632–10633 CrossRef CAS PubMed.
- Q. Zhao, et al., Studies on pervaporation characteristics of polyacrylonitrile–b-poly(ethylene glycol)–b-polyacrylonitrile block copolymer membrane for dehydration of aqueous acetone solutions, J. Membr. Sci., 2008, 311(1–2), 284–293 CAS.
- Q. F. An, et al., Polyacrylonitrile-block-poly(methyl acrylate) membranes 2: swelling behavior and pervaporation performance for separating benzene/cyclohexane, J. Membr. Sci., 2008, 313(1–2), 60–67 CrossRef CAS.
- M. S. A. Rahaman, A. F. Ismail and A. Mustafa, A review of heat treatment on polyacrylonitrile fiber, Polym. Degrad. Stab., 2007, 92(8), 1421–1432 CrossRef CAS.
- C. Tang, et al., Long-Range Ordered Thin Films of Block Copolymers Prepared by Zone-Casting and Their Thermal Conversion Into Ordered Nanostructured Carbon, J. Am. Chem. Soc., 2005, 127(19), 6918–6919 CrossRef CAS PubMed.
- C. Tang, et al., Well-Defined Carbon Nanoparticles Prepared From Water-Soluble Shell Cross-linked Micelles That Contain Polyacrylonitrile Cores, Angew. Chem., Int. Ed., 2004, 43(21), 2783–2787 CrossRef CAS PubMed.
- C. Tran and V. Kalra, Co-continuous nanoscale assembly of Nafion–polyacrylonitrile blends within nanofibers: a facile route to fabrication of porous nanofibers, Soft Matter, 2013, 9(3), 846–852 RSC.
- N. Nishioka, et al., Thermal decomposition of cellulose/synthetic polymer blends containing grafted products. II. Cellulose/polyacrylonitrile blends, J. Appl. Polym. Sci., 1996, 59(8), 1203–1211 CrossRef CAS.
- F. Béguin, et al., A Self-Supporting Electrode for Supercapacitors Prepared by One-Step Pyrolysis of Carbon Nanotube/Polyacrylonitrile Blends, Adv. Mater., 2005, 17(19), 2380–2384 CrossRef.
- P. Raghavan, et al., Preparation and electrochemical characterization of polymer electrolytes based on electrospun poly(vinylidene fluoride-co-hexafluoropropylene)/polyacrylonitrile blend/composite membranes for lithium batteries, J. Power Sources, 2010, 195(18), 6088–6094 CrossRef CAS.
- P. K. Janert and M. Schick, Phase Behavior of Ternary Homopolymer/Diblock Blends:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Influence of Relative Chain Lengths, Macromolecules, 1997, 30(1), 137–144 CrossRef CAS.
Influence of Relative Chain Lengths, Macromolecules, 1997, 30(1), 137–144 CrossRef CAS. - M. M. Coleman and G. T. Sivy, Fourier transform IR studies of the degradation of polyacrylonitrile copolymers—III, Carbon, 1981, 19(2), 133–135 CrossRef CAS.
- S. N. A. M. Jamil, R. Daik and I. Ahmad, Synthesis and Thermal Properties of Acrylonitrile/Butyl Acrylate/Fumaronitrile and Acrylonitrile/Ethyl Hexyl Acrylate/Fumaronitrile Terpolymers as a Potential Precursor for Carbon Fiber, Materials, 2014, 7(9), 6207–6223 CrossRef.
- I. Catta Preta, et al., Thermal behavior of polyacrylonitrile polymers synthesized under different conditions and comonomer compositions, J. Therm. Anal. Calorim., 2007, 87(3), 657–659 CrossRef CAS.
- J.-S. Tsai and C.-H. Lin, Effect of comonomer composition on the properties of polyacrylonitrile precursor and resulting carbon fiber, J. Appl. Polym. Sci., 1991, 43(4), 679–685 CrossRef CAS.
- Z. Wangxi, L. Jie and W. Gang, Evolution of structure and properties of PAN precursors during their conversion to carbon fibers, Carbon, 2003, 41(14), 2805–2812 CrossRef.
- M. A. Semsarzadeh and A. Molaeei, Thermal reactions and analysis of polyacrylonitrile films, Iran. Polym. J., 1997, 6(2), 113–119 CAS.
- T. J. Xue, M. A. McKinney and C. A. Wilkie, The thermal degradation of polyacrylonitrile, Polym. Degrad. Stab., 1997, 58(1–2), 193–202 CrossRef CAS.
- N. Chatterjee, et al., An XRD characterization of the thermal degradation of polyacrylonitrile, J. Polym. Sci., Part B: Polym. Phys., 1995, 33(12), 1705–1712 CrossRef CAS.
- D. W. van Krevelen and K. te Nijenhuis, Properties of Polymers: Their Correlation With Chemical Structure; Their Numerical Estimation and Prediction From Additive Group Contributions, Elsevier Science, 2009 Search PubMed.
- H. Arita, et al., Chain-mixing behavior at interface between polystyrene brushes and polystyrene matrices, Polym. J., 2013, 45(1), 117–123 CrossRef CAS.
- Q. Lan and U. O. Minnesota, Nanoparticle Dispersions in Polymers: Homopolymer Versus Block Copolymer, University of Minnesota, 2007 Search PubMed.
- X. Chen and J. A. Gardella Jr, Surface Modification of Polymers by Blending Siloxane Block Copolymers, Macromolecules, 1994, 27(12), 3363–3369 CrossRef CAS.
- A.-V. Ruzette and L. Leibler, Block copolymers in tomorrow's plastics, Nat. Mater., 2005, 4(1), 19–31 CrossRef CAS PubMed.
- C. Luo, et al., Crystallization behavior of “wet brush” and “dry brush” blends of PS-b-PEO-b-PS/h-PEO, J. Appl. Polym. Sci., 2009, 113(2), 907–915 CrossRef CAS.
- T. Kowalewski, R. D. McCullough and K. Matyjaszewski, Complex nanostructured materials from segmented copolymers prepared by ATRP, Eur. Phys. J. E: Soft Matter Biol. Phys., 2003, 10(1), 5–16 CrossRef CAS PubMed.
- J. Bae, et al., A unique embossed carbon layer from induced domain alignment in a block copolymer thin film under an electric field, Chem. Commun., 2013, 49(48), 5456–5458 RSC.
| This journal is © The Royal Society of Chemistry 2016 |