(CTA)3[SiW12]–Li+–MMT: a novel, efficient and simple nanocatalyst for facile and one-pot access to diverse and densely functionalized 2-amino-4H-chromene derivatives via an eco-friendly multicomponent reaction in water
Esmayeel Abbaspour-Gilandeh*a,
Mehraneh Aghaei-Hashjinb,
Asieh Yahyazadehc and
Hadi Salemid
aYoung Researchers and Elites Club, Ardabil Branch, Islamic Azad University, Ardabil, Iran. E-mail: abbaspour1365@yahoo.com; Fax: +98 45 33332238
bDepartment of Chemistry, Payame Noor University (PNU), Tehran, Iran
cChemistry Department, University of Guilan, Rasht, Iran
dDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Gava Zang, Zanjan, Iran
First published on 23rd May 2016
Abstract
A simple, facile and highly efficient one-pot synthesis of a pharmaceutically interesting diverse kind of functionalized 2-amino-4H-chromene by a straightforward three-component reaction of an aromatic aldehyde, malononitrile (or ethyl cyanoacetate) and diverse enolizable C–H activated acidic compounds in the presence of a catalytic amount of (CTA)3[SiW12]–Li+–MMT is reported as a novel, environmentally friendly, reusable and promising nanocatalyst reaction in refluxing water. Based on the procedure, it was feasible to synthesize 2-amino-3-cyano-pyrano[3,2-c]chromen-5(4H)-one (4a–4y), 2-amino-3-cyano-pyrano[3,2-c]quinolin-5(4H)-one (6a–6s), 2-amino-3-cyano-7,8-dihydro-4H-chromen-5(6H)-one (8a–8u), 2-amino-3-cyano-pyrano[4,3-b]pyran-5(4H)-one (12a–12f), 2-amino-3-cyano-pyrano[3,2-c]pyridine-6(5H)-one (13a–13f), and 1H-pyrano[2,3-d]pyrimidine-2,4(3H,5H)-dione (14a–14f). The structure of the nanocatalyst was confirmed by various techniques such as IR, SEM, TGA-DTG, EDX, ICP and XRD analyses. In comparison to the conventional methods, the salient features of the present protocol are green reaction conditions, high quantitative yields, short reaction time, high atom economy, low cost, easy isolation of products, and no column chromatographic separation.
Introduction
The usage of heterogeneous catalysts in organic synthesis is an interesting method to access green catalysis, which is compatible with the environment and is of high interest in different processes of chemical transformations which occur by catalysis in heterogeneous conditions.1 In recent years, the synthesis of catalysts based on heteropolyacids and related compounds, due to reasons such as the lack of need for separation through distillation or extraction, has attracted high attention in the synthetic chemistry, technology and pharmaceutical industry fields2 and only partial changes in their selectivity and activity provide the possibility of their reuse.3 Heteropolyacids have attracted high attention due to their unique Keggin structures, strong acidity and solubility in non-polar solutions.4 This class of compounds as excellent and versatile catalysts, due to some interesting features including non-corrosiveness, flexible acidic strength, high reductivity, high hydrolytic and thermal stability, and also low toxicity, can be utilized to catalyze a wide range of homogeneous and heterogeneous catalytic processes.5 Nevertheless, drawbacks such as the problem of separation from the reaction mixture and a low surface area (1–10 m2 g−1) have been encountered with the above-mentioned advantages as difficulties.6 It seems that the heterogenization of heteropolyacids in an insoluble organic and separable matrix with a wide surface extent such as zeolite and clay or by formation of micro- and mesoporous salts of cesium or potassium (Cs2.5H0.5PW or K2.5H0.5PW) makes the increase of access to acid sites and control of the acidic strength of the solid possible.7 Montmorillonite (MMT) is one of the most applicable compounds of layered silicate, and its usage has attracted high attention due to its large specific surface area, high modulus, layered structure, high cation exchange capacity, and chemical and mechanical stability, as well as it having natural resources.8 The building blocks of montmorillonite layers consist of an aluminum octahedral layer [Al(OH6)−3] sandwiched between two tetrahedral silicon–oxygen (Si2O5−2) layers.9 According to the ion exchange property of montmorillonite, the ions of Mg2+ and Fe2+ can be replaced with Al3+. Due to these substitutions, a negative electric charge appears in the lattice, and the unit of positive electrical charge as an alkali cation or earth alkaline such as Na+, K+, Ca+ and Mg2+ can replace itself in the space between layers and the bond in the three-unit layers of montmorillonite are established by these cations.10 Montmorillonite layers with a thickness of approximately 1 nm and lateral dimensions of 30 nm up to several micrometers are known as nanoporous with a regular structure.11 The common phenomenon of the broken bonds of layered silicate causes the formation of hydroxyl groups that can be used for chemical modifications.12 It should be noted that the distribution property of MMT in water increases its basic distance and this swell can be attributed to the cation collection in its interlayers. Although clay soil benefits from a high adsorption capacity, the modification of its structure increases the efficacy of these compounds so that they can react with reactants better.134H-Pyrans and 4H-pyran-annulated heterocyclic scaffolds, namely, 4H-chromene moieties, are the key building block of numerous oxygen-containing heterocyclic natural compounds possessing distinct properties of general interest. This structural motif is broadly represented by several types of alkaloids manifesting diverse biological and pharmacological activities including anti-tumor,14 anti-HIV,15 anti-inflammatory,16 antimicrobial and antifungal,17 and anti-allergenic18 ones, along with anti-neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s Diseases (HD) and a few others.19 Furthermore, many of these functionalized 4H-pyran derivatives demonstrate cancer cell growth inhibitory activity and are surveyed as potential anti-cancer agents.20 Moreover, it was found that natural products containing the chromene moiety can play an important role in synthetic approaches to promising compounds in the fields of biodegradable agrochemicals,21 medicine,22 and the pigments and cosmetics industries.23 These valued features have inspired chemists to simulate such natural designs in developing derived analogues of alkaloids, for instance, arisugacin,24 amarogentin,25 (+)-calanolide A,26 huajiaosimuline and veprisine,27 which all contain the key pyran-annulated pharmacophoric motif (Scheme 1).
Experimental
General
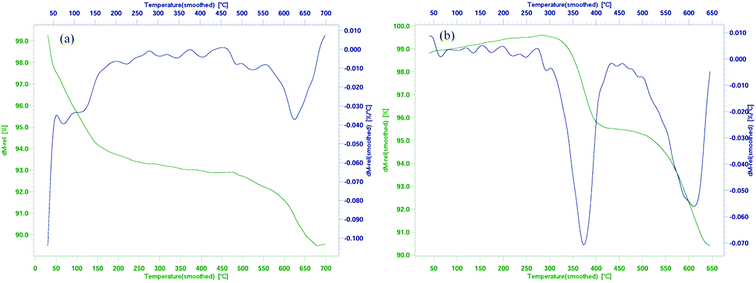
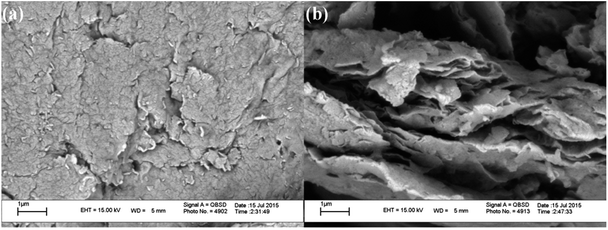
All chemical materials were purchased from Fluka and Merck companies and used without further purification. The purity determination of the product and reaction monitoring were accomplished by TLC on silica gel PolyGram SILG/UV 254 plates. The melting points were determined using an Electrothermal 9100 apparatus and are uncorrected. The IR spectra were recorded as KBr pellets on a PerkinElmer PXI instrument. The NMR spectra were obtained using a BRUKER DRX-300 AVANCE spectrometer at room temperature in DMSO using TMS as an internal standard. Inductively coupled plasma atomic emission spectrometry (ICP-AES) measurements were performed on a VARIAN VISTA-MPX. Thermogravimetric analysis (TGA) was conducted using a Linseis SAT-PT 1000 thermoanalyzer instrument. Samples were heated from 25 to 650 °C at a ramp rate of 10 °C min−1 under N2 atmosphere. Scanning election microscopy (SEM) was carried out on a SEM-LEO 1430VP. The low and broad angle X-ray diffraction (XRD) measurements were accomplished in the 2θ range of 3–80° at room temperature on a Siemens D-500 X-ray diffractometer (Germany), using Ni-filtered Co Kα radiation. Elemental analyses were conducted on a Carlo-Erba EA1110CNNO-S analyzer and agreed (within 0.30) with the calculated values.Catalyst synthesis
Spectral data of the new products
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2195 (CN), 3163 (N–H amidic), 3390, 3454 (N–H amine); 1H NMR (400.22 MHz, DMSO-d6, ppm): δ 5.50 (s, 1H, CH), 7.17 (d, 1H, CH), 7.22 (s, 2H, NH2), 7.34 (dt, 1H, CH), 7.37 (d, 1H, CH), 7.41 (t, 1H, CH), 7.55 (dt, 1H, CH), 7.37–7.57 (m, 2H), 7.80 (d, 1H, CH), 7.95 (dd, 1H, CH), 7.99 (d, 1H, CH), 8.49 (d, 1H, CH); 13C NMR (100.6 MHz, DMSO-d6, ppm): 56.4, 59.9, 109.7, 112.5, 116.0, 1120.4, 122.3, 122.6, 124.5, 125.5, 126.1, 126.3, 126.4, 127.6, 129.0, 131.5, 131.8, 133.9, 138.9, 141.9, 152.1, 159.5, 161.0.
O), 2195 (CN), 3163 (N–H amidic), 3390, 3454 (N–H amine); 1H NMR (400.22 MHz, DMSO-d6, ppm): δ 5.50 (s, 1H, CH), 7.17 (d, 1H, CH), 7.22 (s, 2H, NH2), 7.34 (dt, 1H, CH), 7.37 (d, 1H, CH), 7.41 (t, 1H, CH), 7.55 (dt, 1H, CH), 7.37–7.57 (m, 2H), 7.80 (d, 1H, CH), 7.95 (dd, 1H, CH), 7.99 (d, 1H, CH), 8.49 (d, 1H, CH); 13C NMR (100.6 MHz, DMSO-d6, ppm): 56.4, 59.9, 109.7, 112.5, 116.0, 1120.4, 122.3, 122.6, 124.5, 125.5, 126.1, 126.3, 126.4, 127.6, 129.0, 131.5, 131.8, 133.9, 138.9, 141.9, 152.1, 159.5, 161.0.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2198 (CN), 3174 (N–H amidic), 3325, 3462 (N–H amine); 1H NMR (400.22 MHz, DMSO, ppm): δ 4.90 (s, 1H, CH), 6.94 (dd, 1H, CH), 7.30 (dt, 1H, CH), 7.34 (dd, 1H, CH), 7.36 (d, 1H, CH), 7.41 (s, 2H, NH2), 7.57 (dt, 1H, CH), 7.59 (dt, 1H, CH), 7.89 (dd, 1H, CH), 7.90 (dd, 1H, CH), 11.90 (s, 1H, NH); 13C NMR (100.6 MHz, DMSO, ppm): δ 33.2, 58.1, 110.5, 112.8, 116.0, 120.1, 122.4, 122.6, 125.0, 125.3, 127.5, 132.0, 138.4, 149.4, 151.9, 160.3, 161.0.
O), 2198 (CN), 3174 (N–H amidic), 3325, 3462 (N–H amine); 1H NMR (400.22 MHz, DMSO, ppm): δ 4.90 (s, 1H, CH), 6.94 (dd, 1H, CH), 7.30 (dt, 1H, CH), 7.34 (dd, 1H, CH), 7.36 (d, 1H, CH), 7.41 (s, 2H, NH2), 7.57 (dt, 1H, CH), 7.59 (dt, 1H, CH), 7.89 (dd, 1H, CH), 7.90 (dd, 1H, CH), 11.90 (s, 1H, NH); 13C NMR (100.6 MHz, DMSO, ppm): δ 33.2, 58.1, 110.5, 112.8, 116.0, 120.1, 122.4, 122.6, 125.0, 125.3, 127.5, 132.0, 138.4, 149.4, 151.9, 160.3, 161.0.Results and discussion
Catalyst characterization
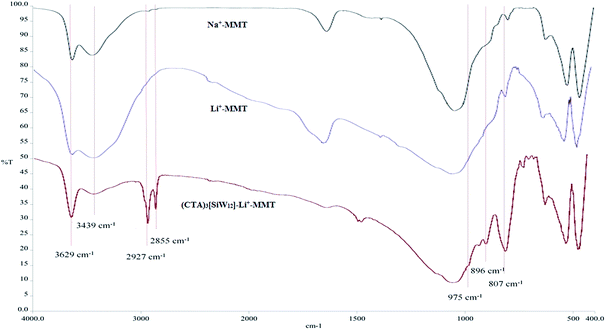
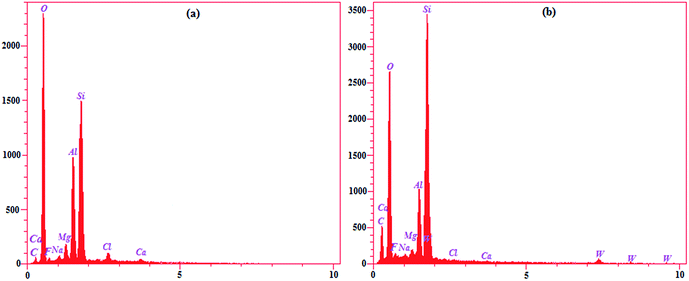
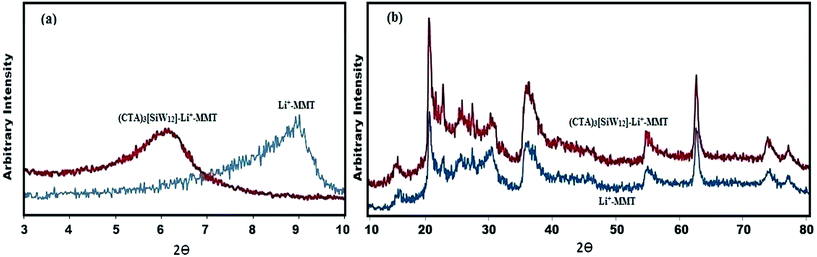
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 896 cm−1 (W–O–W in corner-shared octahedra) and 807 cm−1 (W–O–W in edge-shared octahedra) in the FTIR spectrum. Therefore, despite these peaks, it can be concluded that [SiW12] is surrounded within Li+–MMT.
O), 896 cm−1 (W–O–W in corner-shared octahedra) and 807 cm−1 (W–O–W in edge-shared octahedra) in the FTIR spectrum. Therefore, despite these peaks, it can be concluded that [SiW12] is surrounded within Li+–MMT.
In continuation of our efforts to the synthesis of pyran-annulated compounds and in performing organic transformations with the aid of green synthetic methodologies,29 we decided to investigate the synthesis of 2-amino-3-cyano-4H-pyrans with diverse substituents from the reaction of C–H-activated acid varieties, aldehyde and malononitrile (or ethyl cyanoacetate) in water reflux conditions using (CTA)3[SiW12]–Li+–MMT as a novel, eco-friendly, reusable and promising nanocatalyst (Scheme 2).
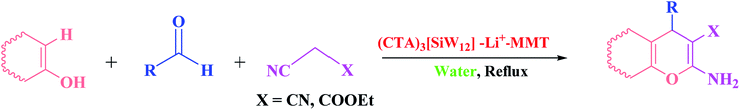 | ||
| Scheme 2 One-pot three-component reaction of different C–H-activated acidic compounds, aldehydes and active methylene nitriles catalyzed by (CTA)3[SiW12]–Li+–MMT in water. | ||
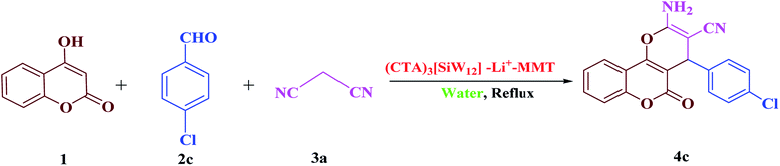
In order to screen the reaction conditions for the synthesis of 2-amino-4-(4-chlorophenyl)-3-cyano-pyrano[3,2-c]chromene-5(4H)-one (4c), a systematic study considering different variables affecting the reaction yield has been investigated in the reaction of 4-hydroxycoumarin (1), 4-chlorobenzaldehyde (2c) and malononitrile (3a) (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1) as the model reaction (Scheme 2). The results are indicated in Table 1. The primary optimization experiments revealed that any formation of the desired product 4c was achieved under solvent-free conditions even at 100 °C (entries 1 and 2). However, the trial reaction in the presence of water as a solvent afforded the desired product 4c in a slightly higher yield compared to the solvent-free conditions (entries 3 and 4). As can be seen in entry 4, the use of reflux conditions improved the yield of the desired product. Unfortunately, the utilization of different solvents such as DMF, DMSO, CH2Cl2, CHCl3 and toluene resulted in low product yields under reflux conditions (entries 5–9). It was found that H2O is the best solvent for the chemical reaction under study. In the next phase of survey, the effect of catalyst loading on the completion of the reaction was evaluated (entries 12–14). After several screening experiments with different amounts of catalyst, it was found that the highest yield (97%) was obtained when a catalytic amount (10 mg) of (CTA)3[SiW12]–Li+–MMT was applied in H2O under reflux conditions (entry 13). Decreasing the amount of catalyst (10 mg to 5 mg) resulted in a lower yield of the product, while increasing the amount of catalyst required for the reaction from 10 mg to 15 mg did not affect the duration of the reaction and the product yield (entries 12 and 14). In addition, to delineate the role of catalyst and temperature effect under solvent-free conditions, the reaction was investigated with and without heating with the same amount of catalyst (10 mg) (entries 10 and 11). It was observed that the use of heating leads to a faster reaction and a higher yield.
1.1) as the model reaction (Scheme 2). The results are indicated in Table 1. The primary optimization experiments revealed that any formation of the desired product 4c was achieved under solvent-free conditions even at 100 °C (entries 1 and 2). However, the trial reaction in the presence of water as a solvent afforded the desired product 4c in a slightly higher yield compared to the solvent-free conditions (entries 3 and 4). As can be seen in entry 4, the use of reflux conditions improved the yield of the desired product. Unfortunately, the utilization of different solvents such as DMF, DMSO, CH2Cl2, CHCl3 and toluene resulted in low product yields under reflux conditions (entries 5–9). It was found that H2O is the best solvent for the chemical reaction under study. In the next phase of survey, the effect of catalyst loading on the completion of the reaction was evaluated (entries 12–14). After several screening experiments with different amounts of catalyst, it was found that the highest yield (97%) was obtained when a catalytic amount (10 mg) of (CTA)3[SiW12]–Li+–MMT was applied in H2O under reflux conditions (entry 13). Decreasing the amount of catalyst (10 mg to 5 mg) resulted in a lower yield of the product, while increasing the amount of catalyst required for the reaction from 10 mg to 15 mg did not affect the duration of the reaction and the product yield (entries 12 and 14). In addition, to delineate the role of catalyst and temperature effect under solvent-free conditions, the reaction was investigated with and without heating with the same amount of catalyst (10 mg) (entries 10 and 11). It was observed that the use of heating leads to a faster reaction and a higher yield.
| Entry | Catalyst | Catalyst loading (mg) | Solvent | Temperature (°C) | Time (min) | Yieldb |
|---|---|---|---|---|---|---|
| a Reaction conditions: 4-hydroxycoumarin (1 mmol), 4-chlorobenzaldehyde (1 mmol), malononitrile (1.1 mmol), water (5 mL) and the required amount of catalyst.b The yields refer to the isolated product. | ||||||
| 1 | — | — | — | r.t. | 6 | — |
| 2 | — | — | — | 100 | 6 | — |
| 3 | — | — | H2O | r.t. | 6 | 15% |
| 4 | — | — | H2O | Reflux | 6 | 33% |
| 5 | — | — | DMF | Reflux | 6 | 18% |
| 6 | — | — | DMSO | Reflux | 6 | 20% |
| 7 | — | — | CH2Cl2 | Reflux | 6 | 17% |
| 8 | — | — | CHCl3 | Reflux | 6 | 17% |
| 9 | — | — | Toluene | Reflux | 6 | 21% |
| 10 | (CTA)3[SiW12]–Li+–MMT | 10 | — | r.t. | 6 | 49% |
| 11 | (CTA)3[SiW12]–Li+–MMT | 10 | — | 100 | 6 | 73% |
| 12 | (CTA)3[SiW12]–Li+–MMT | 5 | H2O | Reflux | 6 | 66% |
| 13 | (CTA)3[SiW12]–Li+–MMT | 10 | H2O | Reflux | 6 | 97% |
| 14 | (CTA)3[SiW12]–Li+–MMT | 15 | H2O | Reflux | 6 | 91% |
In order to generalize the optimum conditions, we extended the reaction of 4-hydroxycoumarin (1) with a range of appropriate aldehydes (2a–t), malononitrile (3a) or ethyl cyanoacetate (3b) under similar conditions (using H2O/(CTA)3[SiW12]–Li+–MMT), furnishing the respective 2-amino-3-cyano-pyrano[3,2-c]chromen-5(4H)-one derivatives (4a–y) in high yields (Scheme 3). The optimized results are listed in Table 2. It was found that aromatic aldehydes with electron-donating groups (entries 7–15 and 25, Table 2) showed relatively low reactivity compared to the electron-withdrawing groups (entries 2–6 and 22–24). In addition to the aromatic aldehydes, the generality and substituent scope of this method were evaluated by employing several heterocyclic aldehydes, obtaining high to excellent yields (entries 16–19, Table 2). It is also remarkable that with malononitrile (3a) a slightly lower reaction time was required compared to ethyl cyanoacetate (3b) (entries 21–25, Table 2).
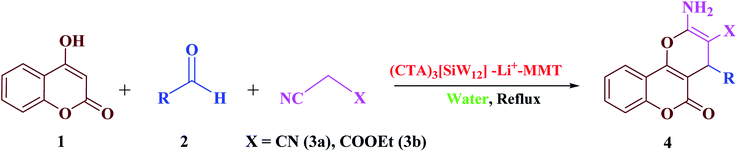 | ||
| Scheme 3 One-pot three-component condensation of 4-hydroxycoumarin (1), various aldehydes (2) and malononitrile or ethyl cyanoacetate (3a–b). | ||
| Entry | RCHO (2) | Carbon acid (3) (X) | Product | Time (min) | Yield (%)c | m.p. (obsd) (°C)b | m.p. (lit) (°C) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 4-hydroxycoumarin (1 mmol), aldehyde (1 mmol), malononitrile or ethyl cyanoacetate (1.1 mmol), water (5 mL, reflux), (CTA)3[SiW12]–Li+–MMT (10 mg).b All compounds are known and their structures were determined from their spectral data and melting points as compared with literature values.c The yields refer to the isolated products. | |||||||
| 4a |  |
3a (CN) | 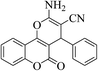 |
9 | 95 | 257–259 | 256–258 (ref. 30a) |
| 4b | 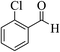 |
3a (CN) | 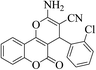 |
6 | 96 | 245–246 | 244–246 (ref. 30b) |
| 4c | 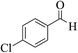 |
3a (CN) | 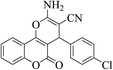 |
6 | 97 | 265–267 | 263–265 (ref. 30l) |
| 4d | 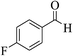 |
3a (CN) | 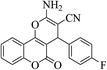 |
4 | 95 | 258–259 | 260–262 (ref. 30a) |
| 4e | 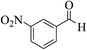 |
3a (CN) | 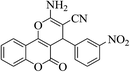 |
8 | 92 | 259–261 | 260–262 (ref. 30c) |
| 4f | 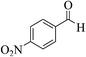 |
3a (CN) | 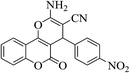 |
8 | 95 | 251–253 | 250–252 (ref. 30c) |
| 4g |  |
3a (CN) | 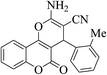 |
10 | 89 | 258–260 | 260–262 (ref. 30a) |
| 4h | 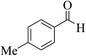 |
3a (CN) | 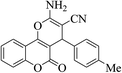 |
10 | 90 | 253–255 | 252–254 (ref. 30a) |
| 4i | 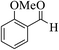 |
3a (CN) | 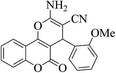 |
15 | 96 | 235–237 | 236–238 (ref. 30h) |
| 4j | 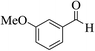 |
3a (CN) | 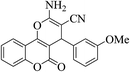 |
12 | 90 | 241–243 | 242–244 (ref. 30h) |
| 4k | 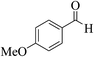 |
3a (CN) | 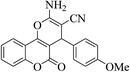 |
12 | 91 | 220–222 | 221–223 (ref. 30b) |
| 4l | 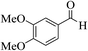 |
3a (CN) | 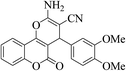 |
10 | 89 | 227–229 | 228–230 (ref. 30a) |
| 4m | 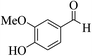 |
3a (CN) | 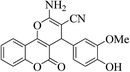 |
12 | 96 | 254–256 | 253–254 (ref. 30j) |
| 4n | 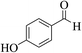 |
3a (CN) | 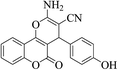 |
10 | 97 | 259–260 | 259–261 (ref. 30e) |
| 4o | 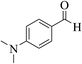 |
3a (CN) | 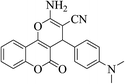 |
15 | 87 | 264–266 | 265–267 (ref. 30a) |
| 4p | 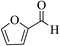 |
3a (CN) | 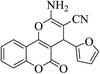 |
6 | 91 | 250–251 | 251–253 (ref. 30d) |
| 4q | 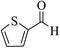 |
3a (CN) | 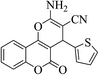 |
6 | 90 | 255–256 | 256–258 (ref. 30m) |
| 4r | 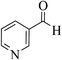 |
3a (CN) | 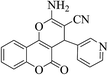 |
15 | 87 | 253–255 | 255–257 (ref. 30c) |
| 4s | 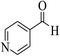 |
3a (CN) | 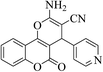 |
15 | 89 | 239–241 | 238–240 (ref. 30k) |
| 4t | 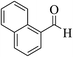 |
3a (CN) | 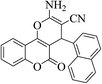 |
8 | 92 | 285–287 | — |
| 4u |  |
3b (COOEt) | 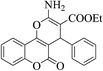 |
20 | 91 | 208–210 | 210–212 (ref. 30f) |
| 4v | 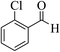 |
3b (COOEt) | 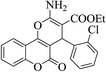 |
15 | 89 | 210–212 | 209–212 (ref. 30g) |
| 4w | 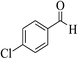 |
3b (COOEt) | 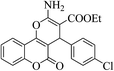 |
18 | 88 | 190–192 | 192–194 (ref. 30h) |
| 4x | 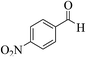 |
3b (COOEt) | 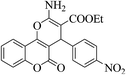 |
10 | 90 | 233–235 | 232–234 (ref. 30i) |
| 4y | 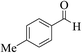 |
3b (COOEt) | 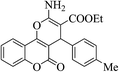 |
20 | 87 | 197–199 | 199–201 (ref. 30i) |
In the following conducted study, and in order to expand the use of this nanocatalyst to other reactions of these categories, a series of poly-functionalized 2-amino-3-cyano-pyrano[3,2-c]quinolin-5(4H)-one derivatives (6a–s) were prepared from an aqueous reaction mixture of 4-hydroxyquinolone (5), different aldehydes (2a–s) and malononitrile (3a) in the presence of a catalytic amount of (CTA)3[SiW12]–Li+–MMT (10 mg) under reflux conditions (Scheme 4). A similar trend of reactivity was obtained for different aldehydes when the reaction was repeated with 4-hydroxyquinolone (5) under the optimized conditions, which is shown in Table 3.
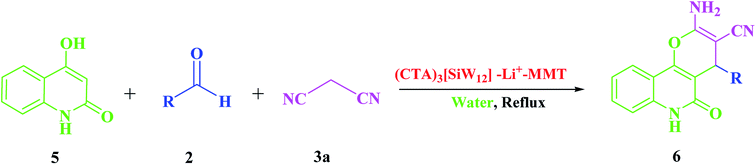 | ||
| Scheme 4 One-pot three-component reaction of 4-hydroxyquinolone (5), different aldehydes (2) and malononitrile (3a). | ||
| Entry | RCHO (2) | Carbon acid (3) (X) | Product | Time (min) | Yield (%)c | m.p. (obsd) (°C)b | m.p. (lit) (°C) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 4-hydroxyquinolin-2(1H)-one (1 mmol), aldehyde (1 mmol), malononitrile or ethyl cyanoacetate (1.1 mmol), water (5 mL, reflux), (CTA)3[SiW12]–Li+–MMT (10 mg).b All compounds are known and their structures were determined from their spectral data and melting points as compared with literature values.c The yields refer to the isolated products. | |||||||
| 6a | 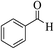 |
3a (CN) | 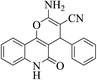 |
10 | 93 | 295–297 | >300 (ref. 31a) |
| 6b | 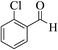 |
3a (CN) | 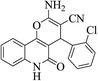 |
8 | 94 | >300 | >300 (ref. 31b) |
| 6c | 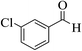 |
3a (CN) | 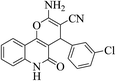 |
6 | 95 | >300 | >300 (ref. 31c) |
| 6d | 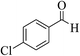 |
3a (CN) | 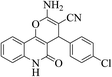 |
8 | 98 | 298–300 | >300 (ref. 31b) |
| 6e | 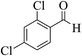 |
3a (CN) | 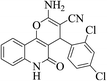 |
5 | 95 | >300 | >300 (ref. 31b) |
| 6f | 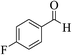 |
3a (CN) | 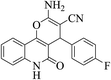 |
7 | 96 | >300 | >300 (ref. 31a) |
| 6g | 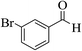 |
3a (CN) | 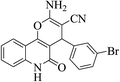 |
8 | 97 | >300 | >300 (ref. 31c) |
| 6h | 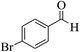 |
3a (CN) | 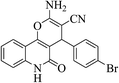 |
6 | 93 | >300 | >300 (ref. 31a) |
| 6i | 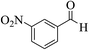 |
3a (CN) | 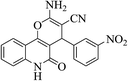 |
9 | 94 | >300 | >300 (ref. 31b) |
| 6j | 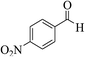 |
3a (CN) | 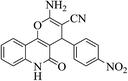 |
12 | 92 | >300 | >300 (ref. 31a) |
| 6k | 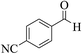 |
3a (CN) | 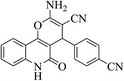 |
12 | 91 | >300 | >300 (ref. 31a) |
| 6l | 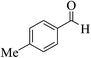 |
3a (CN) | 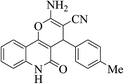 |
13 | 93 | >300 | >300 (ref. 31a) |
| 6m | 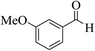 |
3a (CN) | 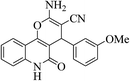 |
12 | 88 | >300 | >300 (ref. 31c) |
| 6n | 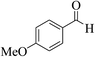 |
3a (CN) | 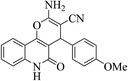 |
12 | 89 | 297–299 | >300 (ref. 31b) |
| 6o | 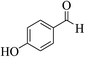 |
3a (CN) | 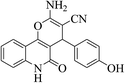 |
13 | 89 | 296–298 | >300 (ref. 31a) |
| 6p | 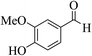 |
3a (CN) | 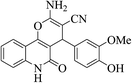 |
12 | 94 | >300 | >300 (ref. 31a) |
| 6q | 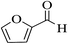 |
3a (CN) | 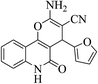 |
7 | 93 | >300 | >300 (ref. 31a) |
| 6r | 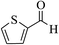 |
3a (CN) | 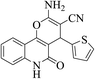 |
7 | 93 | >300 | — |
| 6s | 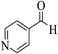 |
3a (CN) | 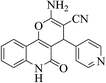 |
13 | 92 | >300 | >300 (ref. 31a) |
After successfully synthesizing a series of 2-amino-3-cyano-pyrano[3,2-c]-quinolin-5(4H)-ones in good to high yields, we turned our attention to the synthesis of 2-amino-3-cyano-7,8-dihydro-4H-chromen-5(6H)-one under similar conditions. We replaced the dimedone compound with 4-hydroxyquinolone under the same conditions (Scheme 5). In this case, the use of dimedone, as an enolic component, improved the reaction time of the desired product 8 slightly (Table 4).
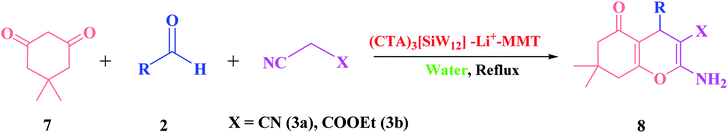 | ||
| Scheme 5 One-pot three-component reaction of dimedone (7), different aldehydes (2) and malononitrile or ethyl cyanoacetate (3a–b). | ||
| Entry | RCHO (2) | Carbon acid (3) (X) | Product | Time (min) | Yield (%)c | m.p. (obsd) (°C)b | m.p. (lit) (°C) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: dimedone (1 mmol), aldehyde (1 mmol), malononitrile or ethyl cyanoacetate (1.1 mmol), water (5 mL, reflux), (CTA)3[SiW12]–Li+–MMT (10 mg).b All compounds are known and their structures were determined from their spectral data and melting points as compared with literature values.c The yields refer to the isolated products. | |||||||
| 8a | 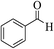 |
3a (CN) | 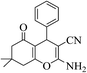 |
15 | 95 | 233–235 | 234–235 (ref. 32d) |
| 8b |  |
3a (CN) | 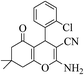 |
15 | 97 | 213–215 | 198–200 (ref. 32b) |
| 8c | 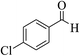 |
3a (CN) | 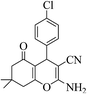 |
12 | 95 | 214–216 | 215–217 (ref. 32a) |
| 8d | 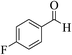 |
3a (CN) | 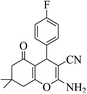 |
14 | 97 | 208–210 | 210–211 (ref. 32d) |
| 8e | 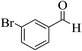 |
3a (CN) | 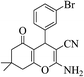 |
17 | 96 | 227–228 | 228–230 (ref. 32j) |
| 8f | 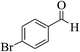 |
3a (CN) | 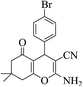 |
12 | 95 | 205–207 | 207–209 (ref. 32i) |
| 8g | 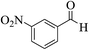 |
3a (CN) | 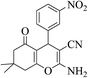 |
11 | 93 | 215–217 | 214–216 (ref. 32a) |
| 8h | 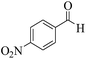 |
3a (CN) | 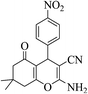 |
11 | 89 | 181–183 | 179–180 (ref. 32c) |
| 8i | 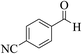 |
3a (CN) | 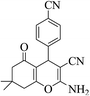 |
13 | 91 | 226–228 | 227–230 (ref. 32i) |
| 8j | 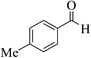 |
3a (CN) | 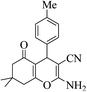 |
20 | 91 | 219–221 | 220–222 (ref. 32e) |
| 8k | 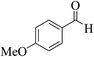 |
3a (CN) | 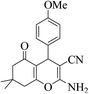 |
22 | 89 | 200–202 | 201–202 (ref. 32d) |
| 8l | 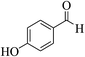 |
3a (CN) | 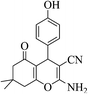 |
19 | 91 | 225–227 | 224–226 (ref. 32d) |
| 8m | 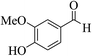 |
3a (CN) | 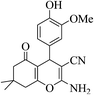 |
27 | 96 | 239–241 | 238–240 (ref. 32f) |
| 8n | 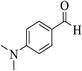 |
3a (CN) | 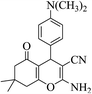 |
19 | 95 | 211–213 | 210–212 (ref. 30g) |
| 8o | 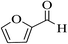 |
3a (CN) | 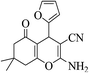 |
12 | 96 | 221–223 | 220–223 (ref. 32g) |
| 8p | 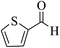 |
3a (CN) | 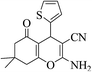 |
15 | 95 | 223–225 | 224–226 (ref. 32h) |
| 8q | 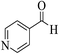 |
3a (CN) | 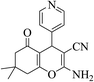 |
15 | 87 | 213–215 | 214–216 (ref. 32k) |
| 8r | 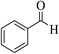 |
3b (COOEt) | 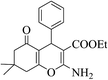 |
45 | 88 | 150–152 | 151–153 (ref. 32e) |
| 8s | 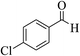 |
3b (COOEt) | 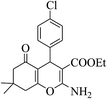 |
29 | 92 | 152–155 | 153–154 (ref. 32e) |
| 8t | 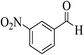 |
3b (COOEt) | 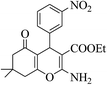 |
32 | 93 | 153–155 | 154–156 (ref. 30g) |
| 8u | 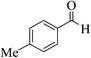 |
3b (COOEt) | 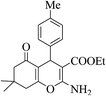 |
45 | 89 | 149–151 | 151–152 (ref. 30g) |
The scope of the present protocol was further surveyed with other C–H-activated acidic compounds such as 4-hydroxy-6-methylpyrone (9), 4-hydroxy-6-methylpyridone (10) and pyrimidine-2,4,6-trione (11). All of the C–H-activated acids 9, 10 and 11 undergo smooth condensation under the reaction conditions providing the desired products, 2-amino-3-cyano-pyrano[4,3-b]pyran-5(4H)-ones (12a–12f), 2-amino-3-cyano-pyrano[3,2-c]pyridine-6(5H)-ones (13a–13f) and 1H-pyrano[2,3-d]pyrimidine-2,4(3H,5H)-diones (14a–14f), respectively in good to high yields (83–97%) within a sensible time frame (Scheme 6). However, the three-component reaction of pyrimidine-2,4,6-trione (11), as a cyclic 1,3-dicarbonyl, required longer reaction times compared to 4-hydroxy-6-methylpyrone (9) and 4-hydroxy-6-methylpyridone (10) under similar reaction conditions (Table 5).
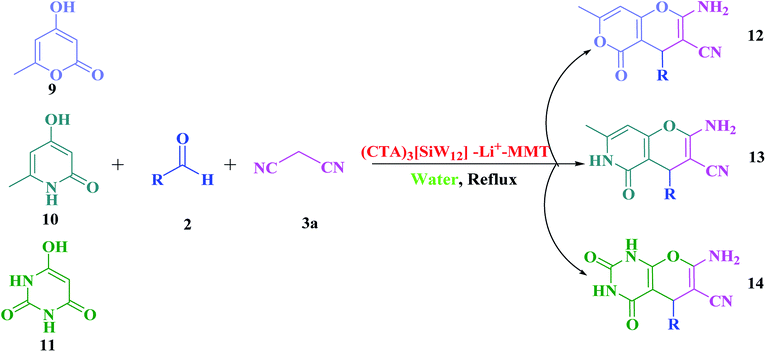 | ||
| Scheme 6 One-pot three-component reaction of C–H-activated acids (9–11), different aldehydes (2) and malononitrile (3a). | ||
| Entry | RCHO (2) | Carbon acid (3) (X) | Product | Time (min) | Yield (%)c | m.p. (obsd) (°C)b | m.p. (lit) (°C) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 4-hydroxycoumarin (1 mmol), aldehyde (1 mmol), malononitrile (1.1 mmol), water (5 mL, reflux), (CTA)3[SiW12]–Li+–MMT (10 mg).b All compounds are known and their structures were determined from their spectral data and melting points as compared with literature values.c The yields refer to the isolated products. | |||||||
| 12a | 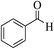 |
3a (CN) | 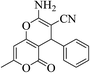 |
18 | 93 | 235–237 | 236–238 (ref. 33a) |
| 12b | 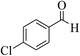 |
3a (CN) | 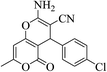 |
15 | 97 | 230–232 | 231–232 (ref. 33b) |
| 12c | 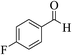 |
3a (CN) | 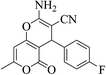 |
15 | 95 | 222–224 | 223–225 (ref. 33c) |
| 12d | 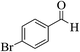 |
3a (CN) | 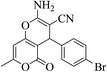 |
15 | 95 | 217–219 | 218–220 (ref. 33c) |
| 12e | 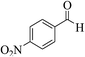 |
3a (CN) | 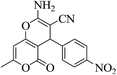 |
15 | 96 | 221–223 | 220–222 (ref. 33d) |
| 12f | 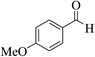 |
3a (CN) | 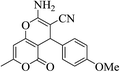 |
20 | 85 | 209–211 | 210–212 (ref. 33e) |
| 13a | 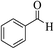 |
3a (CN) | 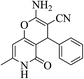 |
20 | 90 | 277–278 | 279–282 (ref. 34) |
| 13b | 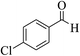 |
3a (CN) | 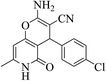 |
18 | 93 | 241–242 | 245–247 (ref. 34) |
| 13c | 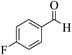 |
3a (CN) | 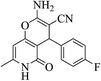 |
18 | 92 | 257–258 | 258–259 (ref. 33c) |
| 13d | 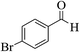 |
3a (CN) | 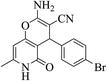 |
18 | 93 | 253–254 | 254–255 (ref. 33c) |
| 13e | 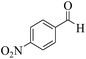 |
3a (CN) | 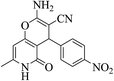 |
15 | 95 | 277–278 | 278–279 (ref. 33c) |
| 13f | 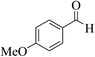 |
3a (CN) | 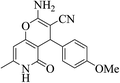 |
18 | 85 | 223–225 | 224–225 (ref. 34) |
| 14a | 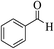 |
3a (CN) | 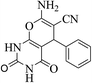 |
35 | 90 | 205–207 | 206–209 (ref. 35a) |
| 14b | 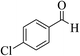 |
3a (CN) | 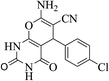 |
30 | 94 | 234–236 | 234–237 (ref. 35b) |
| 14c | 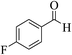 |
3a (CN) | 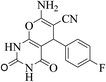 |
30 | 93 | 191–193 | 194–196 (ref. 32k) |
| 14d | 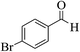 |
3a (CN) | 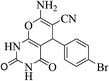 |
30 | 91 | 233–235 | 235–236 (ref. 35c) |
| 14e | 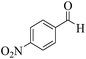 |
3a (CN) | 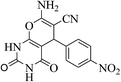 |
25 | 95 | 226–228 | 227–229 (ref. 35b) |
| 14f | 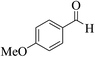 |
3a (CN) | 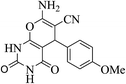 |
35 | 83 | 281–283 | 280 (ref. 35d) |
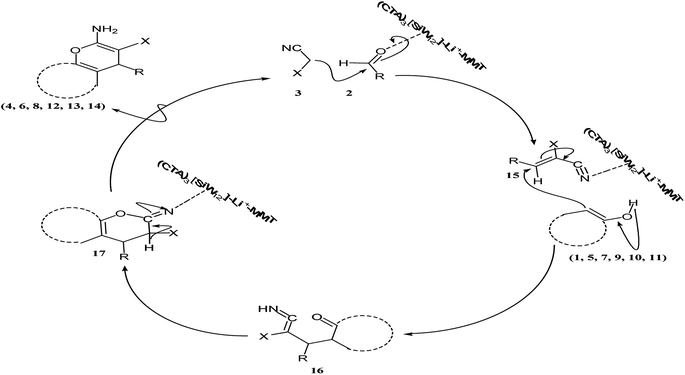
We herein suggest a mechanism in Scheme 7 for the one-pot three-component condensation of various enols, aldehydes and active methylene nitriles catalyzed by (CTA)3[SiW12]–Li+–MMT in water. Firstly, cyanocinnamonitriles or ethyl cyanocinnamates (15) were formed from the Knoevenagel condensation reaction of aldehyde and malononitrile (or ethyl cyanoacetate) in the presence of (CTA)3[SiW12]–Li+–MMT. Then the product of the first step by Michael addition was reacted with the enolate of the C–H-activated acid (1, 5, 7, 9, 10 or 11) in the presence of (CTA)3[SiW12]–Li+–MMT to create a Michael adduct (16). The Michael adduct (16) tautomerizes and cyclizes using the (CTA)3[SiW12]–Li+–MMT catalyst to form an intermediate (17) which then tautomerizes to give the desired product (4, 6, 8, 12, 13 or 14).
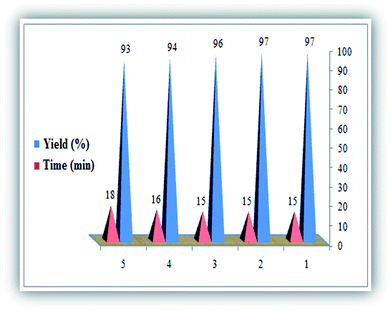
In the next phase of the study, the recovery and reuse cycle of (CTA)3[SiW12]–Li+–MMT was also evaluated. Hence, we investigated the recyclability of (CTA)3[SiW12]–Li+–MMT for five consecutive cycles to afford the synthesis of 2-amino-4-(4-chlorophenyl)-3-cyano-pyrano[3,2-c]chromene-5(4H)-one (4c). As shown in Fig. 6, this nanocatalyst can be recycled at least five times without a significant decrease in the catalytic activity, and the yields ranged from 97% to 93%.
Furthermore, the catalytic efficiency and the capability of (CTA)3[SiW12]–Li+–MMT for some of the obtained results in this study were compared with those of other methodologies which have been reported using other earlier homogeneous and heterogeneous catalysts for the synthesis of different 4H-pyran derivatives (Table 6). It is obvious that a suitable methodology in terms of product yield, reaction time and using a green solvent compared to several of the others has been developed.
| Entry | Catalyst | Catalyst loading | Solvent | Temp (°C) | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | SDS | 20 mol% | H2O | 60 | 150 | 88 | 30a |
| 2 | CuO nanoparticles | 15 mol% | H2O | 100 | 6 | 93 | 36a |
| 3 | DBU | 10 mol% | H2O | 100 | 5 | 94 | 30h |
| 4 | TBAB | 10 mol% | H2O | 100 | 45 | 93 | 30d |
| 5 | POPINO | 5 mol% | H2O | Reflux | 10 | 96 | 36b |
| 6 | (CTA)3[SiW12]–Li+–MMT | 10 mg | H2O | Reflux | 6 | 97 | This work |
| 7 | S-Proline | 10 mol% | H2O/EtOH | 100 | 180 | 78 | 36c |
| 8 | DAHP | 10 mol% | H2O/EtOH | 25 | 240 | 85 | 30g |
| 9 | Urea | 10 mol% | H2O/EtOH | 25 | 420 | 93 | 32k |
| 10 | PBBS | 100 mg | H2O/EtOH | Reflux | 150 | 90 | 36d |
| 11 | TBBDA | 0.18 mmol | H2O/EtOH | Reflux | 170 | 91 | 36d |
| 12 | Hexamethylenetetramine (HMT) | 10 mol% | EtOH | 78 | 40 | 95 | 30j |
| 13 | Nano Al2O3 | 25 mol% | EtOH | 25 | 300 | 89 | 36e |
| 14 | MNP@AVOPc | 20 mg | — | 25 | 15 | 94 | 36f |
| 15 | [Sipim]HSO4 | 0.08 mmol | — | 100 | 30 | 90 | 36g |
Conclusions
In conclusion, we have introduced a simple, facile, green and conveniently practical one-pot methodology for the synthesis of a wide range of biologically and pharmacologically interesting functionalized 2-amino-4H-chromenes in the presence of (CTA)3[SiW12]–Li+–MMT as an eco-friendly and reusable nanocatalyst via a one-pot Knoevenagel condensation of C–H-activated acids, aldehydes and malononitrile (or ethyl cyanoacetate) in water under reflux conditions. The use of a cost-effective and eco-friendly catalyst, aqueous conditions, the absence of hazardous organic solvents, operational simplicity, excellent yields, high atom- economy and avoidance of tedious separation procedures as well as the reusability of the reaction media are the most important advantages of the present method. Considering that the synthetic significance of such densely functionalized and biologically important 2-amino-4H-chromenes scaffolds directly relates to pharmaceutical chemistry, the present strategy with green reaction conditions and easy work-up offers the possibility of its use with affordable and eco-friendly features as appropriate ways for the scale-up of these one-pot three-component reactions.References
- G. A. Olah and A. Molnar, Hydrocarbon Chemistry, John Wiley Sons Inc., New York, 1995 Search PubMed.
- (a) K. M. Su, Z. H. Li, B. W. Cheng, L. Zhang, M. L. Zhang and J. Ming, Fuel Process. Technol., 2011, 92, 2011 CrossRef CAS; (b) D. Carriazo, M. Addamo, G. Marci, C. Martin, L. Palmisano and V. Rives, Appl. Catal., A, 2009, 356, 172 CrossRef CAS; (c) P. Ferreira, I. M. Fonseca, A. M. Ramos, J. Vital and J. E. Castanheiro, Catal. Commun., 2011, 12, 573 CrossRef CAS; (d) A. Micek-Ilnicka, E. Bielanska, L. Litynska-Dobrzynska and A. Bielanski, Appl. Catal., A, 2012, 421, 91 CrossRef; (e) V. V. Costa, K. A. D. Rocha, I. V. Kozhevnikovb and E. V. Gusevskaya, Appl. Catal., A, 2010, 383, 217 CrossRef CAS.
- S. E. Sen, S. M. Smith and K. A. Sullivan, Tetrahedron, 1999, 55, 12657 CrossRef CAS.
- (a) M. Misono, Catal. Rev.: Sci. Eng., 1987, 29, 269 CrossRef CAS; (b) I. V. Kozhevnikov, Catal. Rev.: Sci. Eng., 1995, 37, 311 CrossRef CAS; (c) N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS PubMed.
- (a) T. Okuhara, N. Mizuno and M. Misono, Adv. Catal., 1996, 41, 113 CAS; (b) G. Li, Y. Gu, Y. Ding, H. Zhang, J. Wang, Q. Gao, L. Yan and J. Suo, J. Mol. Catal. A: Chem., 2004, 218, 147 CrossRef CAS.
- D. P. Sawant, A. Vinu, J. Justus, P. Srinivasu and S. B. Halligudi, J. Mol. Catal. A: Chem., 2007, 276, 150 CrossRef CAS.
- (a) Y. Izumi, M. Ogawa and K. Urabe, Appl. Catal., A, 1995, 132, 127 CrossRef CAS; (b) Y. Izumi, K. Hisano and T. Hida, Appl. Catal., A, 1999, 181, 277 CrossRef CAS; (c) S. M. Kumbar, G. V. Shanbhag, F. Lefebvre and S. B. Halligudi, J. Mol. Catal. A: Chem., 2006, 256, 324 CrossRef CAS.
- (a) K. Majdzadeh Ardakani, A. H. Navarchian and F. Sadeghi, Carbohydr. Polym., 2010, 79, 547 CrossRef CAS; (b) S. Kantevari, S. V. N. Vuppalapati and L. Nagarapu, Catal. Commun., 2007, 8, 1857 CrossRef CAS; (c) J. S. Yadav, B. V. S. Reddy, B. Eeshwaraiah and M. Srinivas, Tetrahedron, 2004, 60, 1767 CrossRef CAS; (d) B. S. Kumar, A. Dhakshinamoorthy and K. Pitchumani, Catal. Sci. Technol., 2014, 4, 2378 RSC; (e) F. Shirini, M. Mamaghani and S. V. Atghia, Catal. Commun., 2011, 12, 1088 CrossRef CAS.
- Crystal Structures of clay and their X-ray identification, Monograph, ed. G. W. Brindly and G. Brown, Mineralogical Society, London, 1980, pp. 125–195 Search PubMed.
- E. P. Giannelis, R. Krishnamoorti and E. Manias, Adv. Polym. Sci., 1999, 118, 108 Search PubMed.
- M. Okamoto, S. Mallapragada and B. Narasimhan, Biodegradable Polymer/Layered Silicate Nanocomposites: a review, Handbook of Biodegradable Polymeric Materials and Their Applications, American Scientific Publishers, Valencia, California, 2005, pp. 1–45 Search PubMed.
- (a) H. Shi, T. Lan and T. Pinnavaia, Chem. Mater., 1996, 8, 1584 CrossRef CAS; (b) S. Yariv and H. Cross, in Clays and Clay Minerals, ed. M. Dekker, New York, 2002, pp. 463–566 Search PubMed.
- O. Ozdemir, B. Armagan, M. Turan and M. S. Celik, Dyes Pigm., 2004, 62, 49 CrossRef CAS.
- S. J. Mohr, M. A. Chirigos, F. S. Fuhrman and J. W. Pryor, Cancer Res., 1975, 35, 3750 CAS.
- (a) M. Rueping, E. Sugiono and E. Merino, Chem.–Eur. J., 2008, 14, 6329 CrossRef CAS PubMed; (b) L. Hanna and A. Calanolide, Bulletin of Experimental Treatments for AIDS (BETA), 1998, 12, 8 Search PubMed; (c) M. T. Flavin, J. D. Rizzo, A. Khilevich, A. Kucherenko, A. K. Sheinkman, V. Vilaychack, L. Lin, W. Chen, E. M. Greenwood, T. Pengsuparp, J. M. Pezzuto, S. H. Hughes, T. M. Flavin, M. Cibulski, W. A. Boulanger, R. L. Shone and Z. Q. Xu, J. Med. Chem., 1996, 39, 1303 CrossRef CAS PubMed.
- D. O. Moon, K. C. Kim, C. Y. Jin, M. H. Han, C. Park, K. J. Lee, Y. M. Park, Y. H. Choi and G. Y. Kim, Int. Immunopharmacol., 2007, 7, 222 CrossRef CAS PubMed.
- T. Raj, R. K. Bhatia, A. Kapur, M. Sharma, A. K. Saxena and M. P. S. Ishar, Eur. J. Med. Chem., 2010, 45, 790 CrossRef CAS PubMed.
- (a) L. Bonsignore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517 CrossRef CAS; (b) A. G. Martinez and L. J. Marco, Bioorg. Med. Chem. Lett., 1997, 7, 3165 CrossRef.
- (a) L. L. Andreani and E. Lapi, Bull. Chim. Farm., 1960, 99, 583 Search PubMed; (b) Y. L. Zhang, B. Z. Chen, K. Q. Zheng, M. L. Xu, X. H. Lei and X. B. Yaoxue, Chem Abstr, 1982, 96, 135383e Search PubMed.
- (a) W. Kemnitzer, S. Kasibhatla, S. Jiang, H. Zhang, J. Zhao, S. Jia, L. Xu, C. Crogan-Grundy, R. a. Denis, N. Barriault, L. Vaillancourt, S. Charron, J. Dodd, G. Attardo, D. Labrecque, S. Lamothe, H. Gourdeau, B. Tseng, J. Drewe and S. X. Cai, Bioorg. Med. Chem. Lett., 2005, 15, 4745 CrossRef CAS PubMed; (b) I. S. Chen, S. J. Wu, I. L. Tsai, T. S. Wu, J. M. Pezzuto, M. C. Lu, H. Chai, N. Suh and C. M. Teng, J. Nat. Prod., 1994, 57, 1206 CrossRef CAS PubMed; (c) S. A. Patil, J. Wang, X. S. Li, J. Chen, T. S. Jones, A. Hosni-Ahmed, R. Patil, W. L. Seibel, W. Li and D. D. Miller, Bioorg. Med. Chem. Lett., 2012, 22, 4458 CrossRef CAS PubMed; (d) Y. Gao, W. Yang and D. M. Du, Tetrahedron: Asymmetry, 2010, 23, 339 CrossRef.
- E. A. A. Hafez, M. H. Elnagdi, A. G. A. Elagamey and F. M. A. A. Eltaweel, Heterocycles, 1987, 26, 903 CrossRef CAS.
- (a) A. Shaabani, R. Ghadari, A. sarvary and A. H. Rezayan, J. Org. Chem., 2009, 74, 4372 CrossRef CAS PubMed; (b) M. N. Elinson, A. S. Dorofeev, F. M. Miloserdov, A. I. Ilovaisky, S. K. Feducovich, P. A. Belyakov and G. I. Nikishina, Adv. Synth. Catal., 2008, 350, 591 CrossRef CAS.
- G. P. Ellis, in The Chemistry of Heterocyclic of Compounds. Chromenes, Harmones and Chromones, John Wiley, New York, NY, 1977, ch. II, pp. 11–13 Search PubMed.
- Y. Mehellou and E. D. Clercq, J. Med. Chem., 2010, 53, 521 CrossRef CAS PubMed.
- F. Xuesen, F. Dong, Q. Yingying, Z. Xinying, W. Jianji, M. L. Philippe, A. Graciela, S. Robert and D. C. Erik, Bioorg. Med. Chem. Lett., 2010, 20, 809 CrossRef PubMed.
- N. Kishore, B. B. Mishra, V. Tripathi and V. K. Tiwari, Fitoterapia, 2009, 80, 149 CrossRef CAS PubMed.
- (a) A. Matteelli, A. C. Carvalho, K. E. Dooley and A. Kritski, Future Microbiol., 2010, 5, 849 CrossRef CAS PubMed; (b) K. Schiemann, D. Finsinger, F. Zenke, C. Amendt, T. Knochel, D. Bruge, H. Buchstaller, U. Emde, W. Stahle and S. Anzali, Bioorg. Med. Chem. Lett., 2010, 20, 1491 CrossRef CAS PubMed.
- (a) Y. Q. Zhang, L. H. Gao, K. Z. Wang, H. J. Gao and Y. L. Wang, J. Nanosci. Nanotechnol., 2008, 8, 1248 CAS; (b) K. Z. Wang and L. H. Gao, Mater. Res. Bull., 2002, 37, 2447 CrossRef CAS; (c) J. Gong, X. J. Cui, Z. W. Xie, S. G. Wang and L. Y. Qu, Synth. Met., 2002, 129, 187 CrossRef CAS.
- (a) K. Rad-Moghadam, S. C. Azimi and E. Abbaspour-Gilandeh, Tetrahedron Lett., 2013, 54, 4633 CrossRef CAS; (b) E. Abbaspour-Gilandeh, S. C. Azimi and A. Mohammadi-Barkchai, RSC Adv., 2014, 4, 54854 RSC.
- (a) H. Mehrabi and H. Abusaidi, J. Iran. Chem. Soc., 2010, 7, 890 CrossRef CAS; (b) A. Shaabani, S. Samadi, Z. Badri and A. Rahmati, Catal. Lett., 2005, 104, 39 CrossRef CAS; (c) M. Khoobi, L. Ma’mani, F. Rezazadeh, Z. Zareie, A. Foroumadi, A. Ramazani and A. Shafiee, J. Mol. Catal. A: Chem., 2012, 359, 74 CrossRef CAS; (d) J. M. Khurana and S. Kumar, Tetrahedron Lett., 2009, 50, 4125 CrossRef CAS; (e) W. Xiang-Shan, Z. Zhao-Sen, S. Da-Qing, W. Xian- Yong and Z. Zhi-Min, Chin. J. Org. Chem., 2005, 25, 1138 Search PubMed; (f) M. P. Goncharenko and Y. A. Sharanin, Russ. J. Org. Chem., 1993, 29, 1118 Search PubMed; (g) T. A. Khan, M. Lal, S. Ali and M. M. Khan, Tetrahedron Lett., 2011, 52, 5327 CrossRef; (h) M. J. Khurana, B. Nand and P. Saluja, Tetrahedron, 2010, 66, 5637 CrossRef; (i) T. Shujiang, J. Hong, F. Fang, F. Youjian, Z. Songlei, L. Tuanjie, Z. Xiaojing and S. Daqing, J. Chem. Res., Synop., 2004, 6, 396 Search PubMed; (j) W. Hong-juan, Z. jie and Z. Zhan-Hui, Monatsh. Chem., 2010, 141, 1107 CrossRef; (k) A. A. Shestopalov, L. A. Rodinovskaya, A. M. Shestopalov and V. P. Litvinov, Russ. Chem. Bull., 2005, 54, 992 CrossRef CAS; (l) G. M. Ziarani, A. Badiei, M. Azizi and P. Zarabadi, J. Chem. Eng., 2011, 30, 59 Search PubMed; (m) M. Khoobi, M. Alipour, A. Moradi, A. Sakhteman, H. Nadri, S. F. Razavi, M. Ghandi, A. Foroumadi and A. Shafiee, Eur. J. Med. Chem., 2013, 68, 291 CrossRef CAS PubMed.
- (a) L. Min, L. Ma and L. Hu, Tetrahedron Lett., 2011, 52, 2597 CrossRef; (b) X. Wang, Z. Zeng, D. Shi, X. Wei and Z. Zong, Synth. Commun., 2004, 34, 3021 CrossRef CAS; (c) S. Jadhava, R. patila, D. Kumbhara, A. Patravaleb, D. Chandamb and M. Deshmukha, Int. J. Pharm. Sci. Rev. Res., 2015, 35, 75 Search PubMed.
- (a) M. Kazemzad, A. A. Yuzbashi, S. Balalaie and M. Bararjanian, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2011, 41, 1182 CrossRef CAS; (b) J. K. Rajput and G. Kaur, Catal. Sci. Technol., 2014, 4, 142 RSC; (c) S. Balalaie, M. Bararjanian, A. M. Amani and B. Movassagh, Synlett, 2006, 2, 263 CrossRef; (d) S. Gao, C. H. Tsai, C. Tseng and C. F. Yao, Tetrahedron, 2008, 64, 9143 CrossRef CAS; (e) R. Liangce, L. Xiaoyue, W. Haiying, S. Daqing, T. Shujiang and Z. Oiya, Synth. Commun., 2006, 36, 2363 CrossRef; (f) A. Patra and T. Mahapatra, J. Chem. Res., Synop., 2010, 34, 689 CrossRef CAS; (g) S. Gowravaram, K. Arundhathi, K. B. S. Sudhakar and J. S. Yadav, Synth. Commun., 2009, 39, 433 CrossRef; (h) M. Hong and C. Cai, J. Chem. Res., Synop., 2010, 34, 568 CrossRef CAS; (i) S. Balalaie, M. Bararjanian, M. Sheikh-Ahmadi, S. Hekmat and P. Salehi, Synth. Commun., 2007, 37, 1097 CrossRef CAS; (j) S. Banerjee, A. Horn, H. Khatri and G. A. Sereda, Tetrahedron Lett., 2011, 52, 1878 CrossRef CAS; (k) G. Brahmachari and B. Banerjee, ACS Sustainable Chem. Eng., 2014, 2, 411 CrossRef CAS.
- (a) M. Poliakoff and P. Licence, Nature, 2007, 450, 810 CrossRef CAS PubMed; (b) Green Chemistry: Challenging Perspectives, ed. P. Tundo and P. T. Anastas, Oxford University Press, Oxford, UK, 2000, pp. 79–105 Search PubMed; (c) X. Fan, D. Feng, Y. Qu, X. Zhang, J. Wang, P. M. Loiseau, G. Andrei, R. Snoeck and E. Clercq, Bioorg. Med. Chem. Lett., 2010, 20, 809 CrossRef CAS PubMed; (d) E. V. Stoyanov, I. C. Ivanov and D. Heber, Molecules, 2000, 5, 19 CrossRef CAS; (e) M. Z. Piao and K. Imafuku, Tetrahedron Lett., 1997, 38, 5301 CrossRef CAS.
- S. M. Baghbanian, RSC Adv., 2014, 4, 59397 RSC.
- (a) K. D. Khalil, E. I. Ibrahim and F. A. Al-Sagheer, Catal. Sci. Technol., 2016, 6, 1410 RSC; (b) S. Mashkouri and M. Reza Naimi-Jamal, Molecules, 2009, 14, 474 CrossRef CAS PubMed; (c) G. M. Ziarani, S. Faramarzi, S. Asadi, A. Badiei, R. Bazl and M. Amanlou, Daru, J. Pharm. Sci., 2013, 21, 3 CrossRef PubMed; (d) M. M. Heravi, A. Ghods, K. Bakhtiari and F. Derikvand, Synth. Commun., 2010, 40, 1927 CrossRef CAS.
- (a) H. Mehrabi and M. Kazemi-Mireki, Chin. Chem. Lett., 2011, 22, 1419 CrossRef CAS; (b) M. G. Dekamin, M. Eslami and A. Maleki, Tetrahedron, 2013, 69, 1074 CrossRef CAS; (c) S. Abdolmohammadi and S. Balalaie, Tetrahedron Lett., 2007, 48, 3299 CrossRef CAS; (d) R. Ghorbani-Vaghei, Z. Toghraei-Semiromi and R. Karimi-Nami, J. Braz. Chem. Soc., 2011, 22, 905 CAS; (e) A. Montaghami and N. Montazeri, Orient. J. Chem., 2014, 30, 1361 CrossRef CAS; (f) M. Safaiee, M. A. Zolfigol, F. Afsharnadery and S. Baghery, RSC Adv., 2015, 5, 102340 RSC; (g) K. Niknam and A. Piran, Green Sustainable Chem., 2013, 3, 1 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |