Immobilized ruthenium metal-containing ionic liquid-catalyzed dehydrogenation of dimethylamine borane complex for the reduction of olefins and nitroarenes†
Nilesh M. Patila,
Takehiko Sasaki b and
Bhalchandra M. Bhanage*a
b and
Bhalchandra M. Bhanage*a
aDepartment of Chemistry, Institute of Chemical Technology, N. Parekh Marg, Matunga, Mumbai-400019, India. E-mail: bm.bhanage@gmail.com; bm.bhanage@ictmumbai.edu.in; Fax: +91 22 33611020; Tel: +91 22 33612603
bDepartment of Complexity Science and Engineering, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5, Kashiwanoha, Kashiwa, Chiba 277-8561, Japan
First published on 23rd May 2016
Abstract
An efficient immobilized ruthenium metal containing ionic liquid (ImmRu-IL) catalyst has been developed for the transfer hydrogenation of olefins and nitroarenes. Various olefins and nitroarenes were reduced in excellent yields within 2–6 h at room temperature. This methodology uses eco-friendly dimethylamine borane as a reducing agent which is nontoxic, water soluble, highly stable and easy to handle. The reactions take place through tandem dehydrogenation and hydrogenation of dimethylamine borane complex in the presence of ImmRu-IL catalyst. The catalyst was reused in up to four consecutive cycles without any significance loss in its activity. The fresh and reused catalysts have been studied by XPS analysis.
Introduction
Nowadays, dehydrogenation of ammonia-borane (NH3BH3, AB) and its derivatives like methylamine-borane (CH3NH2BH3, MeAB) and dimethylamine-borane (Me2NHBH3, DMAB) has been gaining great attention1 because of their high hydrogen (H2) storage capacity which could solve the problem of hydrogen economy.2 Several organic and inorganic materials such as metal–organic frameworks (MOF),3 organic materials4 and metal-hydrides5 have been employed for the storage of hydrogen. However, the uses of these materials are limited by (i) the need of high pressure systems, (ii) the need for high temperatures, (iii) low storage H2 capacity and (iv) their difficulty to handle. On the other hand, the ammonia-borane family has great advantages compared to the above mentioned materials because of their high H2 storage capacity, high stability and nontoxicity. In addition, they are easy to handle and are highly soluble in water at room temperature.6 Ammonia-borane and its analogs play vital roles in the polymer industry,7 materials chemistry,8 tandem dehydrogenation–hydrogenation reactions9 and transfer hydrogenation.10 In recent decades, cobalt-catalyzed transfer hydrogenations of unsaturated organic moieties have been achieved by the borane family.11 In particularly, dehydrogenations of dimethylamine-borane (Me2NHBH3, DMAB) are known using various transition-metal catalysts such as ruthenium, iridium and palladium.12 Berke and co-workers have reported homogenous Re-catalyzed alkenes hydrogenation using DMAB as a hydrogen source.13 Chirik's explored homogeneous system of titanium precursor complex [(η5-C5H3-1,3-(SiMe3)2)2Ti]2(μ2,η1,η1-N2) for dehydrogenation of DMAB.14 Of note, Williams and co-workers have described homogenous copper and ruthenium complexes for dehydrogenation of DMAB and in situ reduction of various organic moieties.15 In spite of their potential utility of these protocols, the use of homogeneous non recyclable catalytic systems and expensive phosphine ligands, harsh reaction conditions, longer reaction time and difficulty in catalyst-product separation have limited the applicability of these protocols. In additions to these limitations, it needs tedious preparation of homogeneous catalytic systems. Therefore, there is a need to develop a better cost-effective, heterogeneous, and reusable catalytic system for the dehydrogenation of DMAB.The transition-metal catalyzed hydrogenation or transfer hydrogenation of olefinic bonds and the reduction of nitroarenes are one of the most significant reactions in organic chemistry.16 Hydrogenations of olefin bonds have been carried out in the presence of transition metals such as palladium,17 ruthenium,18 rhodium19 and iridium.20 Beller and co-workers have documented heterogeneous iron and cobalt based catalytic systems for the reduction of nitroarenes using molecular hydrogen.21 Among the available reduction process, the transfer hydrogenation is the most attractive process because it does not require high pressure reaction vessel (autoclave) or use of flammable hydrogen gas. Nowadays, AB and DMAB have been become popular reducing agent due its high hydrogen realizing capacity, highly soluble in water and non-toxicity.22 Yurderi et al. studied Ru(0) NPs catalyzed olefins reduction using DMAB as hydrogen source.23 The bimetallic catalytic systems such as NiPd graphene-supported and CoPd alloy and Au NPs for the reduction of nitroarenes using AB as hydrogen source are also known.24
Immobilization of metals on various supports is important in heterogeneous catalysis as it could solve the problem of catalyst recycling and product separation thereby making process greener and cost-effective.25 From this aspect, eco-friendly ionic liquids immobilized on solid materials have been use for many organic transformations.26 In continuation of our interest in to develop efficient heterogeneous and environmentally benign protocols for the hydrogenation reactions,27 herein we explore a immobilized ruthenium metal-containing ionic liquid [ImmRu-IL] for the transfer hydrogenation of olefins and nitroarenes using DMAB as hydrogen source at room temperature (Scheme 1).
Experimental
Materials
3-Trimethoxysilylpropyl chloride (97+%), N-methylimidazole (99+%), dried redistilled 1-methylimidazole (99+%) and RuCl3·3H2O were purchased form Aldrich and all the dry solvents were obtained from WAKO commercially. Aerosil 300 (300 m2 g−1) was acquired from Japan Aerosil Co. and calcined at 573 K for 1.5 h in air for 30 min in vacuum. The catalyst loading was calculated by XRF measurements using Philips PW2402 XRF spectrometer (X-ray source 40 kV, 100 mA). The XPS of ImmRu-IL was measured using a PHI5000 Versa Probe with monochromatic focused (100 μm × 100 μm) Al Kα X-ray radiation (15 kV, 30 mA) and dual beam neutralization using a combination of argon ion gun and electron irradiation.Catalyst synthesis (ImmRu-IL)
In a dry flask 3-trimethoxysilylpropyl chloride (0.690 mol) and N-methylimidazole (0.690 mol) were added under a nitrogen condition and the resulting reaction mass refluxed for 48 h. After completion of reaction, the reaction mass was allowed to cool at room temperature and then washed with dry ethyl acetate. The obtained residue (1-methyl-3-(3-trimethoxysilylpropyl) imidazolium chloride) was dried at room temperature under reduced pressure for 48 h and then stored at 253 K under dry nitrogen. 1-Methyl-3-(3-trimethoxysilylpropyl) imidazolium chloride (weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and silica (Aerosil 300, surface area 300 m2 g−1, calcined at 573 K for 1.5 h in air) were dispersed in dry toluene and then the reaction mixture was refluxed for 48 h under nitrogen atmosphere. After the completion of reaction, material was filtered on glass filter and finally washed with hot dichloromethane to remove excess of ionic liquid. The obtained solid material is denoted as Imm-IL.
1) and silica (Aerosil 300, surface area 300 m2 g−1, calcined at 573 K for 1.5 h in air) were dispersed in dry toluene and then the reaction mixture was refluxed for 48 h under nitrogen atmosphere. After the completion of reaction, material was filtered on glass filter and finally washed with hot dichloromethane to remove excess of ionic liquid. The obtained solid material is denoted as Imm-IL.
The 3.00 g of Imm-IL and 0.6721 g of RuCl3·3H2O were refluxed in 50 mL of acetonitrile for 24 h. Then solvent was removed using glass filter, followed by washing with acetone several times. The obtained catalyst denoted as ImmRu-IL, was dried by evacuation at room temperature for 24 h. The metal loading of catalyst was 5.82 wt% as determined by XRF measurements (Scheme 2).
Results and discussion
Optimization study for transfer hydrogenation of styrene
The reaction conditions were optimized for the in situ reduction of styrenes using ImmRu-IL catalyst via tandem dehydrogenation of DMAB. For this purpose, styrene (0.5 mmol) was chosen as a model substrate in the presence of ImmRu-IL as a catalyst (Table 1).| Entry | Catalyst | Catalyst loading (mol%) | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: styrene (0.5 mmol), ImmRu-IL (2.5 mol%), (CH3)2NHBH3 (1 equiv.), solvent (2 mL), RT, time (2 h).b Yield based on GC and GC-MS analysis. | ||||
| Catalyst screening and catalyst loading | ||||
| 1 | No catalyst | — | Toluene | 00 |
| 2 | Ru/C (5%) | 2.5 | Toluene | 42 |
| 3 | ImmRu-IL | 2.5 | Toluene | 99 |
| 4 | ImmRu-IL | 1 | Toluene | 59 |
| 5 | Ru-IL | 2.5 | Toluene | 98 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Effect of solvent | ||||
| 6 | ImmRu-IL | 2.5 | Toluene | 99 |
| 7 | ImmRu-IL | 2.5 | Water | 30 |
| 8 | ImmRu-IL | 2.5 | THF | 45 |
| 9 | ImmRu-IL | 2.5 | Methanol | 64 |
| 10 | ImmRu-IL | 2.5 | Ethanol | 69 |
| 11 | ImmRu-IL | 2.5 | — | 96 |
At the beginning, the reaction was carry out in the absence of catalyst, the reaction did not proceed (Table 1, entry 1). Next, we compared the activity of synthesized ImmRu-IL with commercially available Ru/C (5%) and it was found that ImmRu-IL catalyzed furnished excellent of yield of desired product than Ru/C (Table 1, entries 2 and 3). It was found that the 2.5 mol% of ImmRu-IL catalyst provided the corresponding ethyl benzene in highest yield (Table 1, entries 3 and 4). Without silica supported Ru-IL also provided good yield of the desired product (Table 1, entry 5). In the next set of experiments, we screened various non-polar and polar solvents such as toluene, water, THF, methanol and ethanol and it was observed that non polar toluene gave excellent yield of desire product (Table 1, entries 6–10). We have performed the model reaction in absences of solvent and 96% yield of ethyl benzene was observed (Table 1, entry 11). Thus, the optimized reaction conditions for transfer hydrogenation of styrene are: styrene (0.5 mmol), DMAB (1 equiv.), and ImmRu-IL (3 mol%) and toluene as a solvent at room temperature.
With optimized conditions in hand, we extend the developed protocol for the transfer hydrogenation of various olefins using DMAB. The several unsaturated compounds were reduced to the saturated compounds in excellent yields within 2–6 h at room temperature (Table 2).
| Entry | Substrate | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: olefins (0.5 mmol), ImmRu-IL (2.5 mol%), (CH3)2NHBH3 (1 equiv.), toluene (2 mL), RT, time (2–6 h).b Yield based on GC and GC-MS analysis. | ||||
| 1 |  |
 |
2 | 99 |
| 2 |  |
 |
2 | 99 |
| 3 | 1-Decene | N-Decane | 2 | 96 |
| 4 | 1-Hexene | N-Hexane | 2 | 99 |
| 5 |  |
 |
4 | 82 |
| 6 | 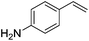 |
 |
6 | 86 |
| 7 |  |
 |
6 | 85 |
| 8 |  |
 |
6 | 84 |
| 9 | 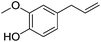 |
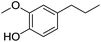 |
6 | 80 |
| 10 |  |
 |
6 | 90 |
| 11 |  |
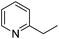 |
6 | 88 |
Styrene was reduced to ethyl benzene with a yield of >99% in 2 h (Table 2, entry 1). Furthermore, cyclohexene, aliphatic 1-decene and 1-hexene were providing good yields of the desired saturated products (Table 2, entries 2–4). Next we also check present protocol for hydrogenation of p-CH3 and p-amino styrene and it was found both substrates provided good yield of the desired products (Table 2, entries 5 and 6). Allylbenzene and substituted allylbenzene were easily reduced under optimized conditions (Table 2, entries 7 and 8). The biomass derived eugenol was successfully reduced in corresponding product with excellent yield (Table 2, entry 9). Furthermore, we have extended our protocol for the reduction of bulky and heterocyclic olefins and it was found that both substrate provided excellent yield of the desired products (Table 2, entries 10 and 11).
Further, the present methodology was used for the transfer hydrogenation of nitroarenes under the optimized conditions. A series of nitro compounds could be reduced into corresponding amines in excellent yields within 2–6 h. Interestingly, the reactions works well even at room temperature without forming any side products (Table 3).
| Entry | Substrate | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: nitroarenes (0.5 mmol), ImmRu-IL (2.5 mol%), (CH3)2NHBH3 (3 equiv.), toluene (2 mL), RT, time (2–6 h).b Yield based on GC and GC-MS analysis. | ||||
| 1 |  |
 |
2 | 99 |
| 2 |  |
 |
6 | 86 |
| 3 | 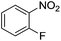 |
 |
6 | 82 |
| 4 |  |
 |
6 | 70 |
| 5 |  |
 |
4 | 86 |
| 6 |  |
 |
4 | 90 |
| 7 |  |
 |
4 | 92 |
| 8 | 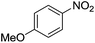 |
 |
5 | 86 |
| 9 | 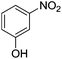 |
 |
2 | 96 |
| 10 |  |
 |
2 | 97 |
| 11 |  |
 |
6 | 85 |
| 12 |  |
 |
6 | 80 |
| 13 |  |
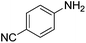 |
6 | 84 |
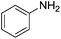
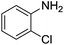
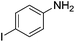
Nitrobenzene cleanly reduction into aniline with excellent yield in 2 h (Table 3, entry 1). Next, the halogen-substituted chloro, fluoro and iodo nitroarenes were studied and it was found that in all the halogenated nitrobenzenes reduced very easily into respective primary amines with good yield (Table 3, entries 2–4). The –NO2 groups with o-, m- and p-methyl and p-methoxy nitrobenzene could also be reduced into related amine successfully (Table 3, entries 5–8). The present catalytic system also works well for nitro phenol reduction within 2 h (Table 3, entries 9 and 10). The reduction of nitro benzyl alcohol and 2-nitroaniline was employed providing products 2-aminobenzyl alcohol and o-phenylenediamine in good yields (Table 3, entries 11 and 12). Furthermore, chemoselectively only nitro groups was reduced when the 4-nitrobenzonitrile was employed under the optimized reaction conditions (Table 3, entry 13).
Recycle study
In an effort to make the present catalytic system more economical and greener, we focused on reusability of ImmRu-IL catalyst for reduction reaction. As shown in Fig. 1 catalyst was recycled for standard reaction of styrene in the presence of DMAB as a hydrogen donor. After completion of reaction, ImmRu-IL catalyst was isolated by filtration method. The filtrate catalyst was washed with toluene several times to remove the rest of organic residue. The recovered catalyst dried in oven and reused for next reaction. The catalyst was recycled up to four consecutive cycles without losing its activity (Table 4).| Sr. no. | Catalyst | H2 source | Recycle study | T (°C) | t (h) | Ref. |
|---|---|---|---|---|---|---|
| a ND: not demonstrated. | ||||||
| 1 | [Ru(p-cymene)Cl2]2 | Me2NHBH3 | ND | 70 | 24 | 15 |
| 2 | Cu(OTf)2 | Me2NHBH3 | ND | 85 | 24 | 15 |
| 3 | Re complex | Me2NHBH3 | ND | 85 | 1–4 | 13 |
| 4 | Pd/KCC-1-NH2 | HCOOH | Recycle | 100 | 6–24 | 16a |
| 5 | RuNPs/ZIF-8 | Me2NHBH3 | Recycle | 40 | 16 | 23 |
| 6 | ImmRu-IL | Me2NHBH3 | Recycle | RT | 2–6 | This work |
XPS study
XPS spectra were measured for the fresh ImmRu-IL, the 1st recycle, and the 4th recycle catalysts to identified elemental composition and valence states of the catalyst. Pollini reported binding energy values for RuCl3 at 285.40 eV (3d3/2) and 281.0 eV (3d5/2), respectively.28 Therefore, the fresh catalyst can be assigned as Ru3+ state. Ru0 exhibits 284.20 eV for 3d3/2, and 279.01–280.20 eV for 3d5/2.29 Main component of the 1st recycle and the 4th recycle samples remain at Ru3+ state with a slight component of reduced Ru0 state, corresponding to the decrease in the intensity of Cl 2p signal for the 1st recycle and the 4th recycle samples (Fig. 2).Conclusions
In summary, we have developed a simple, environmentally benign and recyclable catalytic system for transfer hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and nitro compounds by ImmRu-IL as a versatile heterogeneous catalyst. Various olefins and nitroarenes were reduced via dehydrogenation of DMAB at room temperature. The use of DMAB as hydrogen source makes the developed methodology environmentally benign and nontoxic. The ImmRu-IL catalyst was easily recovered by filtration method and could be reused up to four consecutive cycles without loss of its catalytic activity.
C bonds and nitro compounds by ImmRu-IL as a versatile heterogeneous catalyst. Various olefins and nitroarenes were reduced via dehydrogenation of DMAB at room temperature. The use of DMAB as hydrogen source makes the developed methodology environmentally benign and nontoxic. The ImmRu-IL catalyst was easily recovered by filtration method and could be reused up to four consecutive cycles without loss of its catalytic activity.
General procedure of ImmRu-IL catalyzed transfer hydrogenation of olefins
The olefins (0.5 mmol) and ImmRu-IL catalyst (2.5 mol%) were mixed in 2 mL of toluene in a oven dried Schlenk tube and then DMAB (1 equiv.) was added. Then the reaction mixture was stirred at room temperature for 2–6 h. The progress of reaction was monitored by gas chromatography (GC) and GGMS. All products are well-known in the literature and were confirmed by GC (Perkin Elmer, Clarus 400) (BP-10 GC column, 30 m × 0.32 mm ID, film thickness 0.25 mm) and GCMS (Shimadzu GC-MS QP 2010) analysis.Characterisation data of products
13C NMR (126 MHz, CDCl3) δ 143.77, 129.74, 127.78, 115.24, 20.45.
13C NMR (126 MHz, CDCl3) δ 150.35, 133.80, 120.13, 114.42, 100.17.
Acknowledgements
The author Nilesh. M. Patil is thankful to the University Grant Commission (UGC-SAP), India for providing the Senior Research Fellowship (SRF). XPS analysis was performed at the Research Hub for Advanced Nano Characterization, the University of Tokyo, supports by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This work has been supported by JSPS and DST under the India-Japan Science Cooperative Program (Project No. DST/INT/JSPS/P-152/2013).Notes and references
- A. Staubitz, A. P. M. Robertson and I. Manners, Chem. Rev., 2010, 110, 4079–4124 CrossRef CAS PubMed.
- J. Turner, Science, 1999, 285, 687–689 CrossRef CAS PubMed.
- N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 300, 1127–1129 CrossRef CAS PubMed.
- G. A. Deluga, J. R. Salge, L. D. Schmidt and X. E. Verykios, Science, 2004, 303, 993–997 CrossRef CAS PubMed.
- S.-i. Orimo, Y. Nakamori, J. R. Eliseo, A. Zuttel and C. M. Jensen, Chem. Rev., 2007, 107, 4111–4132 CrossRef CAS PubMed.
- M. E. Bluhm, M. G. Bradley, R. Butterick, U. Kusari and L. G. Sneddon, J. Am. Chem. Soc., 2006, 128, 7748–7749 CrossRef CAS PubMed.
- J. T. Clark, K. Lee and I. Manners, Chem.–Eur. J., 2006, 12, 8634–8648 CrossRef PubMed.
- D. Jacquemin, C. Lambert and E. A. Perpete, Macromolecules, 2004, 37, 1009–1015 CrossRef CAS.
- C. A. Jaska and I. Manners, J. Am. Chem. Soc., 2004, 126, 2698–2699 CrossRef CAS PubMed.
- L. Shi, Y. Liu, Q. Liu, B. Wei and G. Zhang, Green Chem., 2012, 14, 1372–1375 RSC.
- J. K. Pagano, J. P. W. Stelmach and R. Waterman, Dalton Trans., 2015, 44, 12074–12077 RSC.
- C. A. Jaska, K. Temple, A. J. Lough and I. Manners, J. Am. Chem. Soc., 2003, 125, 9424–9434 CrossRef CAS PubMed.
- Y. Jiang and H. Berke, Chem. Commun., 2007, 3571–3573 RSC.
- D. Pun, E. Lobkovsky and P. J. Chirik, Chem. Commun., 2007, 3297–3299 RSC.
- (a) D. van der Waals, A. Pettmanb and J. M. J. Williams, RSC Adv., 2014, 4, 51845–51849 RSC; (b) T. D. Nixon, M. K. Whittlesey and J. M. J. Williams, Tetrahedron Lett., 2011, 52, 6652–6654 CrossRef CAS.
- (a) Z. S. Qureshi, P. B. Sarawade, M. Albert, V. D'Elia, M. N. Hedhili, K. Kohler and J.-M. Basset, ChemCatChem, 2015, 7, 635–642 CrossRef CAS; (b) H. K. Kadam and S. G. Tilve, RSC Adv., 2015, 5, 83391–83407 RSC.
- C. Schmoger, A. Stolle, W. Bonrath, B. Ondruschka, T. Keller and K. D. Jandt, ChemSusChem, 2009, 2, 77–89 CrossRef PubMed.
- C. Pranckevicius, L. Fan and D. W. Stephan, J. Am. Chem. Soc., 2015, 137, 5582–5589 CrossRef CAS PubMed.
- M. E. Sloan, A. Staubitz, K. Lee and I. Manners, Eur. J. Org. Chem., 2011, 672–675 CrossRef CAS.
- Y. Himeda, N. Onozawa-Komatsuzaki, S. Miyazawa, H. Sugihara, T. Hirose and K. Kasuga, Chem.–Eur. J., 2008, 14, 11076–11081 CrossRef CAS PubMed.
- (a) R. V. Jagadeesh, A. E. Surkus, H. Junge, M. M. Pohl, J. Radnik, J. Rabeah, H. M. Huan, V. Schunemann, A. Bruckner and M. Beller, Science, 2013, 342, 1073–1076 CrossRef CAS PubMed; (b) F. A. Westerhaus, R. V. Jagadeesh, G. Wienhofer, M.-M. Pohl, J. Radnik, A.-E. Surkus, J. Rabeah, K. Junge, H. Junge, M. Nielsen, A. Brückner and M. Beller, Nat. Chem., 2013, 5, 537–543 CrossRef CAS PubMed.
- (a) E. Vasilikogiannaki, I. Titilas, G. Vassilikogiannakis and M. Stratakis, Chem. Commun., 2015, 51, 2384–2387 RSC; (b) Q. Yang, Y.-Z. Chen, Z. U. Wang, Q. Xu and H.-L. Jiang, Chem. Commun., 2015, 51, 10419–10422 RSC.
- M. Yurderi, A. Bulut, M. Zahmakiran, M. Gulcan and S. Ozkar, Appl. Catal., B, 2014, 160–161, 534–541 CrossRef CAS.
- (a) H. Goksu, S. F. Ho, O. Metin, K. Korkmaz, A. M. Garcia, M. S. Gultekin and S. Sun, ACS Catal., 2014, 4, 1777–1782 CrossRef CAS; (b) H. Goksu, H. Can, K. Sendil, M. S. Gultekin and O. Metin, Appl. Catal., A, 2014, 488, 176–182 CrossRef CAS; (c) E. Vasilikogiannaki, C. Gryparis, V. Kotzabasaki, I. N. Lykakis and M. Stratakis, Adv. Synth. Catal., 2013, 355, 907–911 CrossRef CAS.
- (a) T. Sasaki, C. Zhong, M. Tada and Y. Iwasawa, Chem. Commun., 2005, 2506–2508 RSC; (b) T. Sasaki, M. Tada, C. Zhong, T. Kume and Y. Iwasawa, J. Mol. Catal. A: Chem., 2008, 279, 200–209 CrossRef CAS.
- (a) M. V. Khedkar, T. Sasaki and B. M. Bhanage, ACS Catal., 2013, 3, 287–293 CrossRef CAS; (b) M. V. Khedkar, T. Sasaki and B. M. Bhanage, RSC Adv., 2013, 3, 7791–7797 RSC; (c) K. V. Wagh, K. C. Badgujar, N. M. Patil and B. M. Bhanage, Curr. Org. Chem., 2016, 20, 736–751 CrossRef CAS.
- (a) N. M. Patil, M. A. Bhosale and B. M. Bhanage, RSC Adv., 2015, 5, 86529–86535 RSC; (b) N. M. Patil, T. Sasaki and B. M. Bhanage, ACS Sustainable Chem. Eng., 2016, 4, 429–436 CrossRef CAS; (c) N. M. Patil, T. Sasaki and B. M. Bhanage, Catal. Lett., 2014, 144, 1803–1809 CrossRef CAS; (d) N. M. Patil and B. M. Bhanage, Catal. Today, 2015, 247, 182–189 CrossRef CAS; (e) D. B. Bagal, Z. S. Qureshi, K. P. Dhake, S. R. Khan and B. M. Bhanage, Green Chem., 2011, 13, 1490–1494 RSC.
- I. Pollini, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 2095–2103 CrossRef CAS.
- NIST X-ray Photoelectron Spectroscopy Database, http://srdata.nist.gov/xps/Default.aspx.
Footnote |
| † Electronic supplementary information (ESI) available: GC-MS, 1H NMR and 13C NMR data of all products. See DOI: 10.1039/c6ra09785e |
| This journal is © The Royal Society of Chemistry 2016 |




