Gold-catalyzed formation of indole derivatives from 2-alkynyl arylazides and oxygen-containing heterocycles†
Abstract
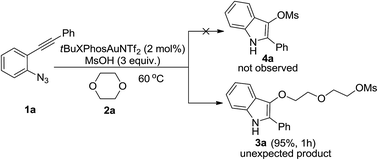
A novel method for the formation of indole derivatives via gold-catalyzed tandem reactions of 2-alkynyl arylazides and oxygen-containing heterocycles has been developed. A variety of indole derivatives were prepared under mild reaction conditions.
- This article is part of the themed collections: Elegant Synthetic Routes to Indole Derivatives and Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...