Ammonium ion detection in solution using vertically grown ZnO nanorod based field-effect transistor
Abstract
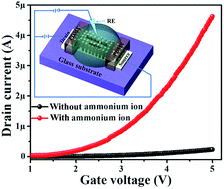
Vertically aligned ZnO nanorods were directly grown on a seeded glass substrate between a pre-deposited source–drain to fabricate a field-effect transistor (FET) based ammonium ion sensor. Controlled growth of aligned nanorods provided a well-defined large surface area for the detection of ammonium ions in solution.

 Please wait while we load your content...
Please wait while we load your content...