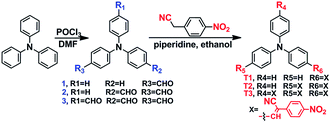
Branched triphenylamine-core compounds: aggregation induced two-photon absorption†
Xin Zhang‡
a,
Xiaoping Gan‡ab,
Shun Yaoa,
Weiju Zhua,
Jianhua Yua,
Zhichao Wua,
Hongping Zhou*a,
Yupeng Tiana and
Jieying Wua
aCollege of Chemistry and Chemical Engineering, Anhui University and Key Laboratory of Functional Inorganic Materials Chemistry of Anhui Province, Hefei 230601, P. R. China. E-mail: zhpzhp@263.net; Fax: +86-551-63828106; Tel: +86-551-63828150
bSchool of Science, Anhui Agricultural University, 230036 Hefei, P. R. China
First published on 13th June 2016
Abstract
Three branched molecules (T1–T3) were synthesized through simple step to realize or enhance two-photon absorption (2PA) in aggregates. Spectra data show that one- and two-branched molecules (T1 and T2) possessed remarkable AIE properties, which came from the formation of J-aggregation because of their partial planarization in an aggregated state. SEM and DLS illustrate that the ordered aggregation and the small particle size had an important influence on fluorescence emission. Open aperture Z-scan experiments show that T1 and T2 possessed excellent 2PA properties in the aggregated state. The largest 2PA cross section was 8314 GM for T2 in aggregates, which was about 13-fold higher than that in pure solution. All the results demonstrate that the compounds could obtain outstanding 2PA performance by rationally adjusting their structure or changing state, which could provide a reference for preparing strong 2PA compounds.
Introduction
Organic compounds with excellent two-photon absorption (2PA) properties are very important for their applications such as optoelectronic materials and biosensors for metal ions, anions and neutral small molecules in cells and tissues,1–3 which have contributed to a greater focus on two-photon biological fluorescent probes and commercial applications of confocal laser two-photon microscopes.4–6 In most cases, typical π–π stacking interactions often lead to aggregation-caused quenching which seriously limits their applications.7,8 Hence, aggregation-induced emission (AIE) or aggregation-induced enhanced emission (AIEE) were proposed by Tang and co-workers to overcome the disadvantages of ACQ,9,10 which has attracted the interest of researchers.11–13 Thus, a few 2PA materials with AIE or AIEE effects have been synthesized, which show good two-photon bioimaging ability in cells. For example, Tian’s group reported a series of heterocycle replaced 1,4-bisstyrylbenzenes functionalized with a cyano group in the styrene double bond to produce steric hindrance which then activated AIE performance.14 These compounds possessed moderate 2PA cross sections (σ) in aggregates in a mixed solution of THF/H2O, which made them potential materials for biophotonic applications. Wu and co-workers reported a series of amino-substituted styrene derivatives that showed good AIE performance in a DMSO/H2O mixed solution and appropriate two-photon excited fluorescence (TPEF).15 Consequently, these compounds were applied in two-photon bioimaging. However, the number of reported 2PA compounds with AIE or AIEE properties is very low, and the structure–activity relationships are not clear. Moreover, their 2PA properties are slightly weak perhaps due to the contradiction between the planarity of 2PA molecules and the twisted conformations of AIE or AIEE molecules. So the design and synthesis of a strong 2PA molecule possessing AIE or AIEE properties is key in two-photon bioimaging and further research is still required.It is well known that branched molecules usually display a synergistic enhancement effect on 2PA properties, while they are not required for the strict planar structure.16,17 Based on the above, we selected triphenylamine as the molecule core to construct branched molecules due to its excellent electron donating ability (D) and variable star-shaped structure.18 Meanwhile, we utilized p-nitrobenzene as an electron acceptor (A) to form an A–π–D model structure at each branch. The donor and acceptor were connected by a carbon–carbon double bond as a π-bridge, then we introduced a cyano group to the double bond as a multi-functional group due to its bulky volume and abundant π–electron density.19 Moreover, the cyano group is also an outstanding electron withdrawing group, which could adjust the molecular dipole moment and enhance intramolecular charge transfer (ICT).20
Herein, linear A–π–D (T1), V-shaped ((A–π–)2D) (T2) and Y-shaped ((A–π–)3D) (T3) molecules (Scheme 1) have been synthesized, using only a two-step reaction to give the final products in high yields (over 90%). All compounds are soluble in common organic solvents such as tetrahydrofuran (THF), acetone, ethyl acetate and dimethyl formamide (DMF) but insoluble in water. We systematically researched their one- and two-photon optical properties in pure organic solution and water–organic mixed solution, which showed that T1 and T2 both possessed superior AIE performance in ethanol/water and DMF/water solutions, respectively. Moreover, T1 and T2 both possessed excellent 2PA properties in an aggregated state; we successfully demonstrated two-photon bioimaging applications based on their favorable biocompatibility.
Results and discussion
Synthesis
Compounds 1, 2 and 3 were prepared according to a reported method.21 Then the condensation of 4-nitrophenylacetonitrile with the respective intermediates 1, 2 and 3 by the Knoevenagel reaction gave the target compounds (T1–T3). All compounds were characterized by 1H, 13C NMR spectroscopy and mass spectrometry (shown in the ESI, Fig. S1†).One-photon absorption and emission properties
The one-photon absorption and fluorescence emission spectra of T1 in five solvents of varying polarity are depicted in Fig. 1. All the corresponding spectroscopic data are collated in Table S1.† It can be seen from Fig. 1a that T1 has two absorption peaks located at ∼300 nm and ∼430 nm, the former is assigned to the π–π* electronic transition caused by the triphenylamine core22 whereas the latter is likely ascribed to intramolecular charge transfer between the triphenylamine core and the terminal group. From Fig. S2 and S3,† it can be seen that the other two compounds have similar features in their absorption spectra. | ||
| Fig. 1 Linear absorption spectra (a) and fluorescence spectra (b) of T1 in five organic solvents of different polarity at a concentration of 5 × 10−5 mol L−1. | ||
The fluorescence spectrum of T1 (Fig. 1b) exhibits one emission peak located between 548 nm and 593 nm in solvents of different polarity, which was assigned to ICT emission.23 As shown in Table S1† and Fig. 1b, upon increasing the solvent polarity, the fluorescence maxima red shifted from 548 nm to 593 nm with a relevant solvatochromic effect. From Fig. S2 and S3,† it can be seen that the other two compounds had a similar feature in their fluorescence spectra. Large Stokes shifts were observed for the three compounds in the five solvents due to strong solvent–solute dipole–dipole interactions, which is a manifestation of the large dipole moment and orientational polarizability. Increased dipole–dipole interactions between the solute and solvent generated a lower energy level. Stokes shifts were approximately proportional to the orientational polarizability.24,25 To consider the effect of solvent on the fluorescence spectra, Lippert–Mataga plots26 for T1–T3 are given in Fig. S4† and the calculation data are collated in Table S2.† The Lippert–Mataga equation was as follows:
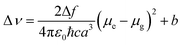 | (1) |
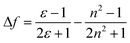 | (2) |
As shown in Fig. S4,† the slopes of the fitted line for T1–T3 are as high as 1.7 × 104, 3.1 × 104 and 1.9 × 104, respectively. The large slopes show large dipole moment changes for these compounds with photoexcitation,27 which was consistent with the significant solvatochromic effect measured experimentally. However, the slope of T3 is smaller than that of T2, the reason perhaps being that T3 has a larger Onsager radius due to its bulky molecular volume.
Aggregation-induced emission
Considering that the three compounds are insoluble in water but soluble in organic solvents, we recorded the absorption and emission spectra of T1–T3 in organic/organic–water mixtures with different water fractions (fw, the volume percentage of water in the organic–water mixtures, that can subtly adjust the solvent polarity). This is revealed by the images shown in Fig. 2 and 3. Absorption spectra of T1 were recorded and are shown in Fig. 2a. We can see that T1 shows two absorption peaks in ethanol which are located at ∼293 nm and ∼441 nm. Meanwhile, the spectra of the mixtures with high fw values start to show level-off tails in the long wavelength region caused by the Mie scattering effect, which implies the formation of nanoaggregates.28 Moreover, the two absorption peaks of T1 and T2 both red shift with increasing fw values, which is a feature of J-aggregation.29 As can be seen from Fig. 3a, T2 in organic–water mixtures also shows similar absorption spectral characteristics to with T1.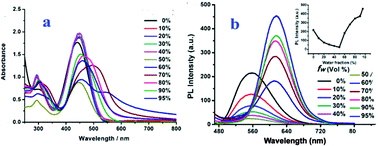 | ||
| Fig. 2 Absorption (a) and fluorescence (b) spectra of T1 in water/ethanol mixtures with fw at 5.0 × 10−5 M. The inset depicts the changes in fluorescence intensity with different fw values. | ||
 | ||
| Fig. 3 Absorption (a) and fluorescence (b) spectra of T2 in water/DMF mixtures with fw at 5.0 × 10−5 M. The inset depicts the changes in fluorescence intensity with different fw values. | ||
The fluorescence spectra of T1 in water–ethanol mixtures with different water fractions are shown in Fig. 2b, which indicate obvious AIE properties when fw ≥ 50%. The fluorescence intensity primarily decreases with increasing fw when fw ≤ 50%, then increases with increasing fwwhen fw ≥ 50%. The fluorescence intensity primarily decreased and the emission peak red shifted slightly with increasing fw when fw ≤ 50%, which could be attributed to twisted intramolecular charge-transfer (TICT),30,31 then the fluorescence intensity increased and the emission peak showed a remarkable red-shift with increasing fw when fw ≥ 50%, which could be ascribed to charge-transfer (CT) excitation32,33 and J-aggregation. The inset depicts the changes in integrated intensity with different water fractions. The fluorescence spectra of T2 in water–DMF mixtures with different water fractions are shown in Fig. 3b. We could see that the fluorescence intensity of T2 was very weak and showed almost no change as fw increases from 0% to 30%, while a significant enhancement in fluorescence intensity was observed in the water–DMF mixtures when fw > 30%, accompanying a small red-shift in the spectrum. The highest fluorescence intensity at fw = 50% was about 17.5-fold higher than that in the pure DMF solution. Subsequently, with increasing the water content, the fluorescence intensity of T2 was slightly decreased. This is also a typical AIE phenomenon but different to that of T1, perhaps because T1 itself possesses weak fluorescence in ethanol solution while T2 possesses no fluorescence emission in pure DMF solution. However, T3 had no AIE effect in various organic solvents mixed with different proportions of water.
To gain further insight into the influence of morphology and particle size on AIE properties, we also carried out scanning electron microscopy (SEM) and dynamic light scatting (DLS) for T1 and T2 at different water fractions. The SEM images of T1 are depicted in Fig. 4 (inset), we can see that aggregates are formed as a needle-like nanoparticles in the mixed solution when fw ≥ 30%, while small globular homogeneous nanoparticles formed immediately in the mixture where fw = 95%, which implies that the polarity of the solvent has an important influence on the morphology of the aggregates. The size distribution of T1 in mixed solutions with different water fractions was conducted by DLS, which showed that the average diameters (d) are 177.2 (fw = 30%), 214.0 (fw = 50%) and 67.8 (fw = 95%) nm (Fig. 4), respectively. The results show that ordered aggregates began to form with the addition of water, the particle size increased when fw ≤ 50% and decreased when fw ≥ 50%, thus leading to a maximum fluorescence intensity at fw = 95%. T2 also shows a similar size distribution influence on AIE properties in mixed solutions of DMF/water at different water fractions. The average diameters (d) of T2 in different water fractions of DMF/water mixtures are 172.4, 72.5 and 220.4 nm (Fig. S5†), respectively. The results agree with the fluorescence intensity changes at different water fractions. All these results illustrate that the particle size of aggregates has an important effect on their AIE properties.
Mechanisms of emission enhancement
In order to better understand the relationship between photophysical properties and molecular structure, single crystals of T1 and T2 were obtained through slow evaporation of methanol and acetonitrile solution, respectively, at room temperature. The crystal structures of the two compounds are shown in Fig. 5 and S6.† Their crystallographic data are summarized in Table S3.† | ||
| Fig. 5 Crystal structures of T1 and T2; (a) the single molecule structure of T1, (b) the 1D linear structure of T1, (c) single molecule structure of T2, and (d) 1D linear structure of T2. | ||
As shown in Fig. 5a and c, the dihedral angle between the plane of the terminal aromatic ring (P1) and the benzene ring (P2) of triphenylamine is 1.858° for T1, and that in T2 is 5.617°, while the dihedral angle of another branch in T2 is 31.55°. The data indicates that T1 possesses good planarity in the solid state except for the other two benzene rings while T2 only had planarity in one branch. Their stacking structures are exhibited in Fig. 5b and d, it can be seen that multiple weak interactions including C8–H8⋯π (ring: C13, C14, C15, C16, C17, C18) (d = 3.12 Å) and C18–H18⋯O3 (d(H18⋯O3) = 2.588 Å, d(C18⋯O3) = 3.246 Å, ∠C18–H18–O3 = 128.17°) play an important role in molecular stacking. Benefiting from these weak interactions and a partially planar structure, T1 and T2 both adopt J-aggregation with a head-to-tail arrangement, which avoids strong π⋯π stacking and makes for fluorescence emission from the aggregated state. To confirm these findings, we simulated the single molecule structure of T1 and T2 in ethanol by theoretical calculations (Fig. 6 and 7).34 Fig. 6 shows that T1 possesses an extremely distorted structure with a large dihedral angle (53.53°) between the planes P1 and P2 in dilute ethanol solution. Compared with that in the crystal state, we could find that molecule aggregation would result in an increment in molecule planarity, which enhances the ICT process and is favourable for the formation of J-aggregation. From Fig. 7, we could see that T2 had similar features in dilute solution and in crystal. Thereby, we could imagine that T3 also had enhanced planarity in crystal but tends not to adopt J-aggregation due to steric hindrance caused by it’s three-branched substituent. In summary, aggregation could enhance molecule planarity and induce the formation of J-aggregation, which could explain the reason why only T1 and T2 possess AIE properties while T3 does not. On the other hand, T3 has three nitro groups which possess strong electron withdrawing ability and always quench fluorescence. As a result, T3 did not display AIE behaviour due to its rather weak fluorescence in the aggregated state and in high polarity solvents such as DMF and ethanol.
Open Z-scan experiments
To investigate the non-linear optical (NLO) properties of T1–T3, we conducted open aperture Z-scan experiments by using a femtosecond laser pulse in pure solution and in aggregates. For Z-scan measurements, the quartz glass cell was 1.0 mm thick, and the average laser power was 500 mW. The results are shown in Fig. 8 and S7† and the experimental data are given in Table 1. The 2PA coefficients (β) for the three compounds were obtained according to eqn (3):
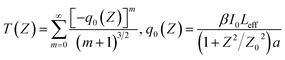 | (3) |
| σ = hνβ × 103/NAd | (4) |
| Compound | T1 | T2 | T3 |
|---|---|---|---|
| Wavelength (nm) | 780 | 820 | 860 |
| σ (GM) in pure solution | N/A | 947 | 756 |
| σ (GM) in aggregates | 4986 | 8314 | 925 |
Cytotoxicity tests
Cytotoxicity is a potential side effect of compounds that must be controlled when dealing with living cells or tissues. Considering their application in cell imaging, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay36 was performed to ascertain the cytotoxic effect of T1–T3 against HepG2 cells over a period of 24 h. Fig. 9 shows the cell viability of HepG2 cells treated with T1–T3 at different concentrations. The results clearly indicate no obvious decrease in cell viability; even when the concentration of the compounds reached up to 50 μM the cell viability was still greater than 90%. The low cytotoxicity of the target compounds over a period of at least 24 h indicates that they are suitable for cellular imaging applications. This is an important factor in further potential live cell imaging applications due to their relatively low cytotoxicity.One- and two-photon fluorescence microscopy cell imaging
To demonstrate the applicability of T1–T3 in cellular imaging, bioimaging experiments were carried out by confocal laser scanning microscopy using HepG2 (human liver cancer cells) as an example. The tested compounds were dissolved in DMSO and then serially diluted in complete culture medium. We initially carried out time-dependent two-photon fluorescence. As a consequence, the three compounds all showed good photo-stability to laser power as time went by, only ∼15% signal deduction was observed (Fig. S8†). The results indicate that these compounds are very suitable for use in biological applications.We then carried out bioimaging studies in living HepG2 cells stained with T1–T3 by both one- and two-photon microscopy. The excitation wavelength was fixed at their maximum absorption wavelength in one-photon microscopy imaging. As shown in Fig. 10a, bright green fluorescence with similar intensity from the cells indicates that T1–T3 could be effectively internalized by HepG2 cells. The three compounds all went through the membrane and localized uniformly in the cytoplasm, which suggests that only the cell cytoplasm could be labelled by these compounds.
Two-photon fluorescence microscopy gives key advantages over one-photon fluorescence imaging, namely, increased penetration depth, lower tissue auto-fluorescence and self-absorption, and reduced photodamage and photobleaching.37 As shown in Fig. 10c, the two-photon microscopy images of HepG2 cells show bright red fluorescence in the cytoplasm, but T2 possesses better two-photon fluorescence imaging ability than T1 and T3, which can be seen from Fig. 10d, the merged pictures of one- and two-photon microscopy. The photograph in Fig. 10d of T2 stained HepG2 cells shows obvious strong yellow fluorescence in the cytoplasm, while there is only partly yellow fluorescence in the cytoplasm stained by T1 and T3. This result could be explained by the reason that the intracellular environment was water soluble which easily led to aggregation of organic compounds, while T1 and T2 possess good AIE performance but T3 has no AIE effect, hence T2 demonstrates excellent two-photon bioimaging performance.
Conclusion
In this work, we have reported a series of branched polar molecules to realize 2PA and improve 2PA in aggregates through forming J-aggregation caused by branched structures which possess partial planarity in one branch in the solid state and fixed steric hindrance in the other two branches. Their excellent 2PA and AIE properties allowed us to successfully apply them in two-photon bioimaging. The structure–property relationships are summarized clearly and all the results show that the branched compounds can also demonstrate outstanding 2PA performance in aggregates by rationally adjusting their structure and changing state, which could provide a reference for designing 2PA compounds.Experimental section
Materials and measurements
Chemicals were purchased and used as received. Every solvent was purified using conventional methods beforehand. 1H, 13C NMR were recorded on 400 MHz and 100 MHz NMR instruments using DMSO as a solvent. Chemical shifts are reported in parts per million (ppm) relative to an internal standard, TMS (0 ppm) and coupling constants reported in Hz. Splitting patterns are described as singlet (s), doublet (d), triplet (t), quartet (q) or multiplet (m). Mass spectra were obtained on a Bruker Autoflex III smart beam mass spectrometer. The X-ray diffraction measurements were performed on a CCD area detector using graphite monochromated MoKα radiation (λ = 0.71069 (Å)) at 298 (2) K. The non-hydrogen atoms were refined anisotropically and hydrogen atoms were introduced geometrically. Calculations were performed using the SHELXTL-97 program package. Dynamic light scattering (DLS) measurements were conducted on a Malvern Zetasizer Nano ZS90 size analyzer. One-photon absorption (OPA) spectra were recorded on a UV-3600 spectrophotometer. One-photon emission fluorescence (OPEF) spectra measurements were performed using a Hitachi F-7000 fluorescence spectrophotometer. The quartz cuvettes used were of a 1 cm path length. The slit pass width of emission spectra was 10 nm, the voltage 500 V. 2PA cross sections (σ) of the samples were obtained by the Open Aperture Z-scan method using a Ti: sapphire system (680–1080 nm, 80 MHz, 140 fs) as a light source.Synthesis
T2 and T3 were obtained as red powders both in over 90% yield by following a similar procedure as for T1. T2: 1H NMR (DMSO-d6, 400 MHz, ppm) δ: 8.33–8.31 (d, J = 8.0 Hz, 4H), 8.21 (s, 2H), 8.01–7.98 (d, J = 8.4, 8H), 7.49–7.45 (t, J = 7.6 Hz, 2H), 7.32–7.29 (t, J = 7.4 Hz, 1H), 7.23–7.21 (d, J = 7.6, 2H), 7.18–7.16 (d, J = 8.0 Hz, 4H). 13C NMR (DMSO-d6, 100 MHz, ppm) δ: 148.88, 146.91, 145.27, 145.09, 140.47, 131.51, 130.24, 127.54, 126.69, 126.50, 126.01, 124.29, 122.56, 117.71, 105.32. MS (m/z): [MH]+, calcd: 590.1750; found 590.1750. T3: 1H NMR (DMSO-d6, 400 MHz, ppm) δ: 8.36–8.34 (d, J = 8.4 Hz, 6H), 8.29 (s, 3H), 8.08–8.02 (q, J = 7.4 Hz, 12H), 7.32–7.30 (d, J = 8.4 Hz, 6H). 13C NMR (DMSO-d6, 100 MHz, ppm) δ: 106.35, 117.55, 124.33, 124.78, 126.75, 128.93, 131.64, 140.35, 145.22, 147.11, 148.17. MS (m/z): [M + H]+, calcd: 762.2023; found 762.9845.
Cytotoxicity assays in cells
To ascertain the cytotoxic effect of the three compounds, the MTT assay was performed. HepG2 cells were trypsinized and plated to ∼70% confluence in 96-well plates 24 h before treatment. Prior to treatment with the compounds, the Dulbecco’s modified eagle medium (DMEM) was removed and replaced with fresh DMEM, and aliquots of the compound stock solutions (1 mM DMSO) were added to obtain final concentrations of 5, 10, 20, 30 and 50 mM. The treated cells were incubated for 24 h at 37 °C under 5% CO2. Subsequently, the cells were treated with 5 mg mL−1 MTT (40 μL per well) and incubated for an additional 4 h (37 °C, 5% CO2). Then, DMEM was removed, the formazan crystals were dissolved in DMSO (150 μL per well), and the absorbance at 570 nm was recorded. The cell viability (%) was calculated according to eqn (5):
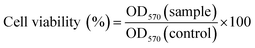 | (5) |
Acknowledgements
This work was supported by The National Natural Science Foundation of China (21271003, 21271004, 51432001 and 51472002), the Science and Technology Plan of Anhui Province (1604b0602016), the Ministry of Education of the People’s Republic of China, and the Higher Education Revitalization Plan Talent Project of Anhui Province (2013).Notes and references
- J. L. Geng, C. C. Goh, N. D. Tomczak, J. Liu, R. R. Liu, L. Ma, L. G. Ng, G. G. Gurzadyan and B. Liu, Chem. Mater., 2014, 26, 1874–1880 CrossRef CAS.
- X. Liu, Y. M. Sun, Y. H. Zhang, F. Miao, G. C. Wang, H. S. Zhao, X. Q. Yu, H. Liu and W. Y. Wong, Org. Biomol. Chem., 2011, 9, 3615–3618 Search PubMed.
- A. R. Morales, A. Frazer, A. W. Woodward, H. Y. A. White, A. Fonari and K. D. Belfield, J. Org. Chem., 2013, 78, 1014–1025 CrossRef CAS PubMed.
- W. Huang, F. S. Tang, B. Li, J. H. Su and H. Tian, J. Mater. Chem. C, 2014, 2, 1141–1148 RSC.
- L. Kong, Y. P. Tian, Q. Chen, Q. Zhang, H. Wang and D. Tan, et al., J. Mater. Chem. C, 2015, 3, 570–581 RSC.
- T. Jiang, Y. Qu, B. Li, Y. Gao and J. Hua, RSC Adv., 2015, 5, 1500–1506 RSC.
- D. D. Li, Y. P. Zhang, Z. Y. Fan, J. Chen and J. H. Yu, Chem. Sci., 2015, 6, 6097–6101 RSC.
- D. Zhang, Y. Gao, J. Dong, Q. Sun, W. Liu, S. Xue and W. Yang, Dyes Pigm., 2015, 113, 307–311 CrossRef CAS.
- X. Zhang, X. Zhang, L. Tao, Z. Chi, J. Xu and Y. Wei, J. Mater. Chem. B, 2014, 2, 4398–4414 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- C. W. T. Leung, Y. Hong, S. Chen, E. Zhao, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 62–65 CrossRef CAS PubMed.
- A. Qin, J. W. Y. Lam and B. Z. Tang, Prog. Polym. Sci., 2012, 37, 182–209 CrossRef CAS.
- Y. Z. Xie, G. G. Shan, P. Li, Z. Y. Zhou and Z. M. Su, Dyes Pigm., 2013, 96, 467–474 CrossRef CAS.
- B. Wang, Y. Wang, J. Hua, Y. Jiang, J. Huang, S. Qian and H. Tian, Chem.–Eur. J., 2011, 17, 2647–2655 CrossRef CAS PubMed.
- Y. Q. Liu, M. Kong, Q. Zhang, Z. Zhang, H. Zhou, S. Zhang and J. Y. Wu, J. Mater. Chem. B, 2014, 2, 5430–5440 RSC.
- M. Rumi, J. E. Ehrlich, A. A. Heikal, J. W. Perry, S. Barlow, T. C. Parker, C. Parker, H. Rockel, S. Thayumanavan, S. R. Marder, D. Beljonne and J. L. Brédas, J. Am. Chem. Soc., 2000, 122, 9500–9510 CrossRef CAS.
- Y. Gao, Y. Qu, T. Jiang, H. Zhang, N. He, B. Li and J. Hua, J. Mater. Chem. C, 2014, 2, 6353–6361 RSC.
- X. Gan, Y. Wang, X. Ge, W. Li, X. Zhang, W. Zhu and Y. Tian, Dyes Pigm., 2015, 120, 65–73 CrossRef CAS.
- B. An, J. Gierschner and S. Park, Acc. Chem. Res., 2012, 45, 544 CrossRef CAS PubMed.
- G. Zhang, M. Aldred, W. Gong, C. Li and M. Zhu, Chem. Commun., 2012, 48, 7711 RSC.
- M. Thomas, G. Said, M. Meziane, B. Mireille and M. Olivier, Synthesis, 2015, 11, 1771–1774 Search PubMed.
- D. D. Li, X. S. Sun, M. M. Wang, H. Yu, H. P. Zhou and J. Y. Wu, Sens. Actuators, B, 2015, 220, 1006–1016 CrossRef CAS.
- H. Detert and V. Schmitt, J. Phys. Org. Chem., 2006, 19, 603–607 CrossRef CAS.
- F. Jin, D. Xu, H. Zhu, Y. Yan, J. Zheng and J. Zhang, et al., Dyes Pigm., 2014, 109, 42–53 CrossRef CAS.
- Z. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899–4032 CrossRef PubMed.
- X. P. Ge, X. P. Gan, Y. Shun, K. Wang, W. J. Zhu and J. H. Yu, J. Mater. Chem. B, 2016, 4, 2785–2793 RSC.
- L. Wang, Z. Zheng, J. Yu, J. Zheng, M. Fang and J. Wu, et al., J. Mater. Chem. C, 2013, 1, 6952–6959 RSC.
- R. Araya, V. Andino-Pavlovsky, R. Yuste and R. Etchenique, ACS Chem. Neurosci., 2013, 4, 1163–1167 CrossRef CAS PubMed.
- Q. Q. Zhao, J. G. Liu, H. Y. Wang, M. G. Li, K. Zhou and H. Yang, et al., J. Mater. Chem. C, 2015, 3, 8183–8192 RSC.
- M. Chen, L. Z. Li, H. Nie, Y. Shi, J. Mei and J. Wang, et al., Chem. Commun., 2015, 51, 10710–10713 RSC.
- M. Chen, H. Nie, B. Song, L. Z. Li, A. J. Qin and B. Z. Tang, J. Mater. Chem. C, 2016, 4, 2901–2908 RSC.
- Y. Zhang, K. Wang, G. Zhuang, Z. Xie, C. Zhang and F. Cao, et al., Chem.–Eur. J., 2015, 21, 2474–2479 CrossRef CAS PubMed.
- (a) X. Du, J. Qi, Z. Zhang, D. Ma and Z. Y. Wang, Chem. Mater., 2012, 24, 2178–2185 CrossRef CAS; (b) Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899–4032 CrossRef PubMed; (c) Z. H. Guo, T. Lei, Z. X. Jin, J. Y. Wang and J. Pei, Org. Lett., 2013, 15, 3530–3533 CrossRef CAS PubMed; (d) X. Y. Shen, Y. J. Wang, E. Zhao, W. Z. Yuan, Y. Liu, P. Lu, A. J. Qin, Y. G. Ma, J. Z. Sun and B. Z. Tang, J. Phys. Chem. C, 2013, 117, 7334–7347 CrossRef CAS.
- Y. Jiang, Y. Wang, J. Hua, J. Tang, B. Li and S. Qian, et al., Chem. Commun., 2010, 46, 4689–4691 RSC.
- Y. Gao, Y. Qu, T. Jiang, H. Zhang, N. He and B. Li, et al., J. Mater. Chem. C, 2014, 2, 6353–6361 RSC.
- Z. Zheng, Q. Zhang, Z. Yu, M. Yang, H. Zhou and J. Wu, et al., J. Mater. Chem. C, 2013, 1, 822–1830 RSC.
- D. Xu, Z. Yu, M. Yang, Z. Zheng, L. Zhu and X. Ye, et al., Dyes Pigm., 2014, 100, 142–149 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S2–S8, Tables S1–S3. CCDC 1437376 and 1428578. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra09701d |
| ‡ These authors contributed equally to this work and should be considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2016 |