Chemically robust solution-processed indium zinc oxide thin film transistors fabricated by back channel wet-etched Mo electrodes†
Da Eun Kim‡
,
Sung Woon Cho‡,
Bora Kim,
Jae Hui Shin,
Won Jun Kang,
Myeong Gu Yun,
Seung Ki Beak,
Hyung Koun Cho*,
Yong-Hoon Kim and
Yunseok Kim
School of Advanced Materials Science and Engineering, Sungkyunkwan University, 300 Cheoncheon-dong, Jangan-gu, Suwon, Gyeonggi-do 440-746, Republic of Korea. E-mail: chohk@skku.edu; Fax: +82 31 290 7410; Tel: +82 31 290 7364
First published on 26th May 2016
Abstract
We designed a systematic processing strategy for solution-processed indium zinc oxide thin film transistors (TFTs) with chemically wet-etched Mo electrodes and chemically durable channels prepared by a sol–gel method. First, we explored the effect of H2O2 wet-etchant pH to define efficiently wet-etched Mo source/drain electrodes without Mo residues and with minimal chemical damage to the indium zinc oxide (IZO) channel. Next, sufficient condensation reaction times and a two-step engineering process were performed on the solution-processed IZO thin films to improve their inferior chemical durability (from incomplete metal oxygen bonds and low film density). The solution-processed IZO channels with wet chemical patterning and superior chemical durability preserved the original electrical transfer properties with minimal electrical degradation in the back channel etch (BCE) processes. Finally, additional N2 post-annealing partially recovered the field-effect mobility (2.5 cm2 V−1 s−1), and on-current without oxidation of the Mo electrode, comparable to the lift-off processed TFTs. This approach provides a significant potential for using wet-based BCE processes in sol–gel prepared oxide TFTs.
Introduction
Amorphous oxide semiconductors (AOSs), specifically those based on indium and zinc oxide materials (such as InGaZnO, InZnO, ZnSnO, In2O3, and ZnO) are considered as typical n-type semiconductor materials for thin film transistors (TFTs) because they have several advantages: superior mobility, transparency to visible light, and high device uniformity over a large area.1,2 However, it can be problematic to pattern the source/drain (S/D) electrodes directly onto the AOS materials because the AOS films are easily etched in the wet-etchant (acid and base solutions)3 and damaged by energetic plasma particles in the dry-etch process.4 Alternatively, etch-stopper layers can be used to protect the AOS film surface (inserted between the AOS and S/D electrodes) for the dry- or wet-etching process of metal electrodes.5 However, the application of an etch-stopper layer can lead to several demerits: an increase of the manufacturing cost due to additional photolithography and mask processes for the etch-stopper layer, an additional parasitic capacitance by the misaligned etch-stopper layer in the short channel region, and the use of plasma and high temperatures in plasma-enhanced chemical vapor deposition for the deposition of the etch-stopper layer.5–7Although AOS channels are readily susceptible to both dry and wet chemical etching, S/D electrodes prepared via wet-etching with a back channel etch (BCE) structure are widely used for mass production. First, to define S/D electrodes in the BCE based TFTs via a wet etching process (without the use of typical etch-stopper layers), we must develop metal oxide semiconductors with superior chemical durability8 and an appropriate wet-etchant with excellent selectivity, etching profile, and no metal residues.9 Recent studies on sputter-deposited AOS TFTs mainly focused on the selection of an appropriate Mo-based wet-etchant rather than improving the chemical durability of sputter-deposited AOS materials.7,9,10 This was mainly because the oxide films grown via sputtering had a superior film density and strongly bonded M–O (metal–oxygen) bonds due to vacuum deposition and post-thermal treatment.9–11
In contrast, solution-processed oxide semiconductors using the sol–gel process (a commonly used technique to fabricate metal oxide TFTs with a low cost, high throughput, and simple processing) have relatively incomplete M–O bonds and a low film density. Despite active studies of solution-processed metal oxide semiconductors (such as metal precursor engineering,12 aqueous route solvent,13 deep ultraviolet photochemical activation,14 and combustion reactions15) all these processes inevitably suffered from incomplete decomposition of the metal precursor ligand-related impurities (e.g., nitrides, acetates), the insufficient conversion of metal precursors to M–O bonds, and a prevalent film porosity in the AOS film. Therefore, solution processed metal oxide semiconductors are expected to show an inferior chemical durability compared to the sputter-deposited metal oxide semiconductor.16 To form an M–O–M lattice via a sol–gel process, metal salt precursors should be fully converted to complete M–O bonds via pyrolysis, hydrolysis, condensation, and densification processes. Meanwhile, insufficient condensation and densification reactions would cause relatively weak M–O bonds and lead to low chemical durability against wet-etchants. In particular, solution-processed oxide semiconductors consisting of In–O and Zn–O based materials (e.g., indium zinc oxide (IZO), ZnO, In2O3, and indium gallium zinc oxide) are susceptible to chemical damages by wet-etchants due to the material's inferior chemical durability compared to zinc tin oxide films.16,17
Generally, single spin-coated and short time-annealed metal oxide semiconductor channel TFTs fabricated using sol–gel methods have shown a good field-effect mobility (μFE ≥ 1 cm2 V−1 s−1) and subthreshold swing (SS), even at a short thermal condensation time of ≤1 h.2,15,18–20 To date, most studies have used un-patterned channels21,22 and the S/D electrode definition by a lift-off process23,24 and shadow mask1,25 in the solution-processed oxide TFTs; thus, the solution processed oxide TFTs did not experience soaking in the acidic or basic solutions. Many researches could ignore the effect of the back channel damage of the AOS channel and the short thermal condensation reaction (400 °C annealing for 1 h) have shown sufficient oxide TFT performances.15,18–20 However, the definition of metal electrodes for industrial mass product and large-area process inevitably requires a wet-chemical etching process. Especially, the amorphous-IZO (a-IZO) semiconductor and Mo metal are very popular channel and electrode materials, respectively, in the solution-processed oxide TFTs.15,26 Furthermore, Mo electrode is suitable to post-annealing process to recover degraded electrical property of solution-processed oxide channel on BCE process due to less metal oxidation, compared to other conventional metal materials (Al and Cu).
In the fabrication of the oxide TFTs using wet-etch patterned Mo electrodes, appropriate wet-etchants should ensure a fast Mo etch rate, superior etch profile, and no Mo-related residues. In addition, the selected wet-etchant must have an extremely low etch rate for metal oxide channel films during the wet-etching process. Thus, oxide channel layers must endure the wet-etchant with superior chemical and electrical durability. Next, appropriate post-annealing process is necessary to recover the electrical damaged performance and surface chemical states on oxide channel layer from wet-etchant without metal oxidation. Consequently, the systematic wet-etching process of Mo electrode is ideally necessary for the fabrication of solution processed IZO TFTs on large-area and mass production.21 Nevertheless, the application of Mo electrodes using a BCE process in the fabrication of solution-processed IZO TFTs has not been investigated. In this work, we developed chemically durable IZO thin films by improving the film density and constructing strong M–O bonds; the etching behavior of a-IZO thin films in the base H2O2 etchant for Mo patterning was vigorously studied.9,10 Finally, the wet-chemically patterned, high performance IZO TFTs were fabricated using the back channel wet-etch process for an Mo electrode without etch-stopper layers.
Experimental
Preparation of the IZO precursor solution
A 0.1 M IZO precursor solution was prepared by dissolving zinc acetylacetonate hydrate [Zn(C5H7O2)2·xH2O] and indium nitrate hydrate [In(NO3)3·xH2O] in 2-methoxyethanol, as metal precursors for the combustion reaction. To determine the appropriate electrical properties of the a-IZO film for the oxide TFTs, the molar ratio of Zn and In in the IZO precursor solution was determined to be 60 and 40%, respectively. The precursor solution was vigorously stirred at 400 rpm for 12 h at room temperature to form a homogeneous and transparent solution. Then, the precursor solution was filtered through a 0.2 μm syringe filter.Sol–gel coating of solution-processed IZO films
The IZO precursor solutions were spin-coated at 3000 rpm for 30 s on a heavily doped p++-Si wafer with a 200 nm thick SiO2 layer. This required a two-step annealing process: the first is a soft-bake at a low temperature (200 °C) and the next step is a hard-bake at a high temperature (400 °C). In the first step, the spin-coated IZO films were soft-baked at 200 °C for 10 min for degassing of the medium complexes from precursor ligands and for the hydrolysis reaction from metal precursor to M–OH bonds. After the soft-bake, a hard-bake was performed to form the M–O bonds via a condensation reaction and to improve the film density at 400 °C on a hotplate in air. To control the chemical durability with the improvement of the film density and the M–O bond strength in the solution-processed IZO films, three types of double stacked IZO layers were prepared (as shown in ESI Fig. S1 and S2†):Fabrication of solution-processed a-IZO TFTs with wet-chemically etched Mo electrodes
A bottom-gate and top-contact structure was adopted for all the TFT fabrication. After the solution-processed IZO layers were deposited onto SiO2 (200 nm)/p++-Si substrates, the IZO channels were wet-patterned in diluted hydrochloric acid by conventional photolithography. Mo (100 nm) electrodes were deposited through sputtering and defined as source/drain electrodes with dimensions of W/L = 500/50 μm via a wet-etching process. The Mo layers were wet-chemically etched in the H2O2-based wet-etchant, which comprises mainly hydrogen peroxide with sulfuric acid and several hydrogen peroxide stabilizers. Here, the pH value of the wet etchant was controlled by adding KOH (pH controller) at 32 °C. The exact etching time was determined from the etch rate in the thick Mo films, and the pattern of Mo electrodes was obtained after sufficient over-etching time (+30 s).Characterization of the IZO thin films and TFTs
The electrical characteristics of the fabricated a-IZO TFTs were measured in the dark at room temperature using a semiconductor parameter analyzer (HP-4145B). The surface morphology and chemical bonding of the a-IZO films were evaluated using optical microscopy (Leica-DH2700), atomic force microscopy (AFM), a spectroscopic ellipsometer (SE MG-1000UV), and angle-resolved X-ray photoelectron spectroscopy (AR-XPS, Theta Probe, Thermo Fisher Scientific Co.) with Al Kα at 1486.6 eV. The relative film porosity of the IZO layers was determined from the refractive index using the Lorentz–Lorenz relation:27
 | (1) |
Results and discussion
Etching behavior of Mo films by a pH-controlled H2O2-based etchant
Typically, H2O2-based and HNO3-based etchants are considered as effective Mo wet-etchants due to their high etching selectivity between the Mo electrode and metal–oxide channel. However, serious chemical damage is caused on the surface of the metal–oxide semiconductor channels by low-pH nitric acid (HNO3)-based etchants (pH < 4). This reduces the electrical performance of the oxide TFTs due to the severe acidic atmosphere compared to those fabricated with H2O2-based etchant (pH ∼ 6).28 To design an adequate Mo wet-etching process for solution-processed a-IZO TFTs with a BCE process, H2O2-based wet-etchants must ensure complete removal of Mo related residues and a rapid Mo etch rate with minimal chemical damage of IZO films. According to the Pourbaix diagram of Mo metal on a standard hydrogen electrode (SHE) vs. 0 V in a water solution at room temperature,29 the formation of various pH-dependent Mo-related phases, including Mo metal, Mo oxide, and Mo-bonded ions, is possible. In particular, the Mo metal can readily be converted to stable Mo oxides in the bare-H2O2 environment (pH ∼ 6):| Mo + H2O2 → MoO2 + H2O | (2) |
It is accepted that the MoO2 phases (Mo oxide) on the back channel of n-type metal oxide film can provide acceptor-like trapping sites for electron carriers, which lead to electrical performance deterioration (on-current and mobility) and electrical instability under repeated positive bias stresses.30 Thus, the formation of these stable MoO2 phases (Mo oxide) should be considered in the a-IZO TFTs processed with bare-H2O2 (pH ∼ 6). In contrast, MoO42− ions become the most stable among various Mo related phases in the base H2O2 solution with alkaline environments (pH > 9), as follows:
| Mo + 2H2O2 → 2H+ + MoO42− + H2↑ | (3) |
It is expected that the Mo metal can be completely ionized to MoO42− without the formation of MoO2, with base H2O2 (pH > 9) wet-etchants. Thus, pH-controlled base H2O2 wet-etchants are preferred (created by adding a prototypical strong base (KOH) to the bare H2O2 wet-etchant) to suppress the formation of an ultrathin MoO2 film on the metal oxide channel surface. A small amount of KOH is able to deliberately increase the pH due to its high dissociation constant (Ka) and does not make particular medium precipitation phases in H2O2 wet-etchant.
First, we investigated the influence of the pH-controlled H2O2 wet-etchant on the etch rate of Mo metal and the removal of the Mo residues. Fig. 1 shows optical microscopy images and X-ray photoelectron spectroscopy (XPS) measurements of Mo metals on SiO2/p++-Si substrate after a sufficient wet-etching time in the pH-controlled H2O2 wet-etchant and Mo etch rate estimated from the thicker Mo electrodes. The etch rate of Mo (100 nm) electrodes on SiO2/p++-Si substrates was considerably accelerated from 2 nm s−1 (pH ∼ 6) to 8.1 nm s−1 (pH ∼ 9.5) by adding KOH to the H2O2 wet-etchant. In particular, even though the Mo was sufficiently wet-etched for over-etching of +30 s in some wet-etchants (pH < 9), the Mo regions with a red color (pH ∼ 6) and dark yellow color (pH ∼ 8.5) were distinctly observed on the SiO2 substrates (Fig. 1a). Here, the exact etching times were estimated with the etching rate from the thicker Mo electrodes. Thus, it is assumed that the remaining Mo is due to a delayed etching rate originating from the formation of strong Mo-related oxide phases. It is also confirmed that these regions consist of the produced Mo oxides, such as MoO2 (binding energy = 229.1 and 232.2 eV) and MoO3 (binding energy = 232.0 and 235.1 eV) via XPS measurements.4
Consequently, these stable Mo oxides dramatically slowed the Mo etch rate by passivating the surface of the Mo films. They degraded the electrical performances of the a-IZO TFTs, despite sufficient over-etching time, due to the formation of acceptor-like trap sites on the IZO back channel, as shown in ESI Fig. S3.† In contrast, by adding KOH to the H2O2 wet-etchants, the Mo etch rate was continuously enhanced and the Mo-related residues were sporadically discovered. Furthermore, the electrical performance of the a-IZO TFTs was simultaneously improved with less Mo-related residues on the IZO back channel. Finally, the residues were completely removed at pH 9.5 with a short over-etching time, as shown in Fig. 1a and b. According to the Pourbaix diagram of Mo in alkaline water solution (pH > 9), it is expected that the Mo would be preferentially ionized to MoO42− in base H2O2 (pH > 9), rather than Mo oxide. No Mo 3d peaks related with the Mo oxides were found in the XPS analysis (Fig. 1b). As a result, we can identify that alkaline H2O2 wet-etchant above pH 9 is adequate to accelerate the etch rate of Mo and to eliminate Mo residues. However, the enhanced alkaline condition of the H2O2 wet-etchant may chemically influence the surface (back-channel) of the sol–gel prepared IZO films via the breakdown and ionization of M–O bonds due to the relatively weak solution processed IZO bonding.
According to the Pourbaix diagram of In and Zn metals in aqueous solution (with respect to pH, at room temperature), it is expected that In2O3 is a more stable phase than other In related phases (In3+, InOH2+, InO2−) at pH 7–11.31 In contrast, the ZnO can be selectively ionized to Zn related ions (Zn2+, ZnOH+, HZnO2−, ZnO22−), except for at a narrow pH region (pH 9–10).32 Thus, the pH 9.5 base H2O2 wet-etchant could be used to suppress chemical damage to IZO thin films and ensure fast Mo etching without Mo residues.
Fabrication of IZO films with a high chemical durability
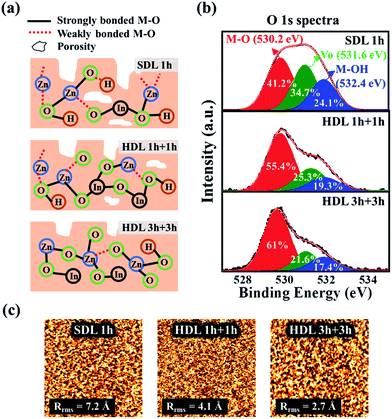
For the wet-chemical processing of Mo electrodes, solution-processed IZO films must endure the attack from the base H2O2 wet-etchants (pH 9.5) with a superior chemical durability. Generally, because they consist of strong M–O bonds and dense film due to physical deposition via sufficient plasma energy, the vacuum-processed metal oxide films using a sputtering method show excellent chemical resistance. In contrast, solution-processed metal oxide films are formed via sequential chemical reactions, such as pyrolysis, hydrolysis, condensation, and densification, from metal precursors at high temperatures. During thermal treatment, most intermediate products (H2O, carbon- and nitrate-related gases) were vaporized out of the films and generated small pores. The metal precursors were transformed to the final M–O bonds via M–OH bonds, which were reinforced via a condensation reaction when there was sufficient reaction energy and time. An insufficient thermal energy supply may produce solution-processed metal oxide films with various incomplete weak M–O bonds, small pores, and metal ligand-related residues, compared to vacuum-processed metal oxide films. These weak M–O bonds formed via an insufficient condensation reaction will be preferentially broken from the base H2O2 wet-etchant (pH 9.5), since they will be ionized to metal ions, and supply dangling bonds on the back channel. Furthermore, pore-sites that exist near the surface and in the bulk regions will significantly extend the active surface area and increase the opportunities for chemical contact between the wet-etchant and metal oxide. Thus, we must suggest a strategy to reinforce the M–O bond and minimize the pore-sites of solution-processed IZO thin films to improve the chemical durability and suppress the wet-etching reaction area. To minimize chemical damage from the wet-etchant, we fabricated solution-processed IZO thin films with strong M–O bonds by providing a sufficient condensation reaction time and superior density by an engineered two-step stacking process. Three samples of solution-processed IZO films controlling stacking process and condensation reaction time were prepared and named as SDL 1 h, HDL 1 h + 1 h, and HDL 3 h + 3 h, as explained in Experimental section (Fig. 2a).The XPS measurements were used to analyze the contribution of oxygen chemical bonds (metal–oxygen, M–O; oxygen deficiency, VO; and metal–hydroxyl group, M–OH) in the samples, as shown in Fig. 2b. Individual O 1s peaks were deconvoluted with Gaussian distributions after subtracting the background, and consisted of M–O, VO, and M–OH centered at 530.2 ± 0.05 eV, 531.6 ± 0.02 eV, and 532.4 ± 0.01 eV, respectively.33,34 Additionally, their bulk volume porosity (Table 1) and surface roughness (Fig. 2c) were compared using ellipsometry and atomic force microscopy (AFM), respectively. The SDL 1 h sample shows severe volume porosity (25.1%) in the film density and poor surface roughness (7.2 Å) due to insufficient by-product degassing and pore refilling. In addition, the high intensity ratio (24.1%) of IM–OH/Itotal (IM–O + IVO + IM–OH) (or the reduced IM–O contribution) is identified from an insufficient condensation reaction time (1 h). In contrast, the HDL 1 h + 1 h sample exhibited relatively improved volume porosity (23.1%) and smooth surface (4.1 Å), compared to the SDL sample. This is ascribed to enough degassing from the bottom layer by two annealing processes (1 + 1 h) and suppression of the pore-sites in the hard-baked bottom layer by an additional top-layer coating, even though the top layer experienced densification process for 1 h. In addition, this sample simultaneously shows the contribution of improved M–O bonding (55.4%) and reduced M–OH ratio (19.3%). Lastly, the IZO film (HDL 3 h + 3 h), which is double-stacked and hard-baked for a prolonged time (3 + 3 h), comparatively reduced the volume porosity (22.7%) and surface roughness (2.74 Å). Consequently, sufficient reaction energy for both the top- and bottom-layers provides the highest ratio of the wanted M–O bond (61%) via the interaction of well-arranged metal and oxygen ions. Thus, it is expected that the solution-processed HDL 3 h + 3 h sample has a satisfactorily chemical durability in the base H2O2 wet-etchant for a Mo wet-etching process, based on high density of strong M–O bonds, superior film density, and smooth surface.
IZO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
Thickness (nm) | n (at 600 nm) | Porosity (volume%) |
|---|---|---|---|
| SDL 1 h | 15.5 | 1.672 | 25.1 |
| HDL 1 h + 1 h | 15.2 | 1.69 | 23.1 |
| HDL 3 h + 3 h | 14.5 | 1.7 | 22.7 |
Etching behavior of sol–gel prepared IZO thin films in the base H2O2 etchant (pH 9.5, 32 °C)
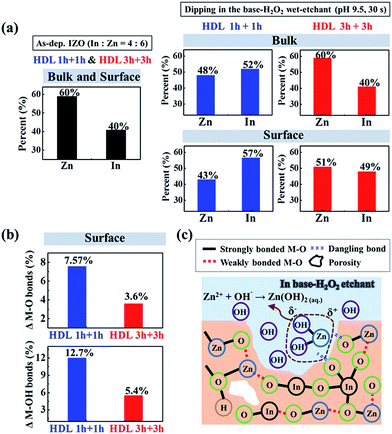
Next, we explore the etching behavior of solution-processed IZO films in the base H2O2 wet-etchant at pH 9.5 and 32 °C using electrochemical dissolution monitoring and angle resolved XPS (AR-XPS) analysis. To distinguish the dissolved chemical elements (In or Zn) from the solution-processed IZO film in the base H2O2 wet-etchant (pH 9.5), time dependent corrosion current densities from the solution-processed In2O3, ZnO, and IZO films (HDL 1 h + 1 h) were obtained at a potential of 0 V vs. SHE in a dark state using a potentiostatic three-electrode cell (see Fig. S4 in the ESI†). Interestingly, the solution-processed In2O3 film shows almost zero corrosion current density and it implies that the etching of the In2O3 film rarely occurs in the base H2O2 wet-etchant (pH 9.5), as shown in Fig. 3a. In contrast, the solution-processed ZnO film shows a significantly higher corrosion current density and this value is quite similar to that from the solution-processed IZO film (HDL 1 h + 1 h). In addition, we confirmed that the SiO2 regions in the partially dipped part of the ZnO and IZO films were clearly observed by the wet etchant, while the In2O3 film showed the absence of the etched boundary (Fig. 3b). Therefore, we can conclude that the Zn–O bonds were favorably broken from solution-processed IZO films dipped in the base H2O2 wet-etchant (pH 9.5), instead of the In–O bonds.We can also verify that the Zn–O bond is preferentially broken on the surface of the IZO film (HDL 1 h + 1 h) with relatively weak M–O bonding compared to the IZO film (HDL 3 h + 3 h). After dipping the HDL 1 h + 1 h and HDL 3 h + 3 h samples in the base H2O2 wet-etchant (pH 9.5) for 30 s, the relative intensity of Zn 2p and In 3d peaks were compared with AR-XPS results for the bulk (normal angle = 20°) and surface (normal angle = 60°) region with varying sample rotation angle.35 Several surveys revealed that the contribution of chemically adsorbed oxygen states (–OH) near the surface of the oxide films was enhanced via AR-XPS measurement.36,37 As shown in Fig. 4a and ESI Fig. S5,† the compositional ratio between In and Zn in the as-dep HDL 1 h + 1 h and HDL 3 h + 3 h samples has an equivalent value in the surface and bulk regions (In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn = 40%
Zn = 40%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60%), similar to the ratio of pristine metal precursors in the sol–gel solution. However, after dipping the samples in the base wet-etchant, the Zn ratio in the HDL 1 h + 1 h sample was anomalously reduced into both bulk (48%) and surface (43%) regions. This indicates that the preferred breakdown of Zn–O bonds actively occurs in the surface region, which is attributed to the preferential etching and large reaction area (high density of pores) of the HDL 1 h + 1 h with the top layer of relatively weak M–O bonding. In contrast, the HDL 3 h + 3 h film reveals a slight decrease in the Zn ratio in the surface (51%), while the bulk (60%) region shows an identical Zn/In ratio to the initial ratio of the as-dep sample. Thus, these results conclusively show that the IZO (HDL 3 h + 3 h) film that consists of strong M–O bonds, less pore-sites, and a smooth surface via a sufficient condensation reaction and efficient double layer stacking process shows superior chemical durability against the base H2O2 wet-etchant. Additionally, the base H2O2 wet-etchant results in an increase of the intensity ratio of the M–OH bond and a decrease of the M–O bond in the surface region of IZO films. In particular, these M–OH bonds are dominantly generated on the surface of the HDL 1 h + 1 h film, where the intensity ratio of the newly generated M–OH bond contributes to about 12.7% and 5.4% from the HDL 1 h + 1 h and HDL 3 h + 3 h, respectively, as shown in Fig. 4b, ESI Fig. S6 and S7.†
60%), similar to the ratio of pristine metal precursors in the sol–gel solution. However, after dipping the samples in the base wet-etchant, the Zn ratio in the HDL 1 h + 1 h sample was anomalously reduced into both bulk (48%) and surface (43%) regions. This indicates that the preferred breakdown of Zn–O bonds actively occurs in the surface region, which is attributed to the preferential etching and large reaction area (high density of pores) of the HDL 1 h + 1 h with the top layer of relatively weak M–O bonding. In contrast, the HDL 3 h + 3 h film reveals a slight decrease in the Zn ratio in the surface (51%), while the bulk (60%) region shows an identical Zn/In ratio to the initial ratio of the as-dep sample. Thus, these results conclusively show that the IZO (HDL 3 h + 3 h) film that consists of strong M–O bonds, less pore-sites, and a smooth surface via a sufficient condensation reaction and efficient double layer stacking process shows superior chemical durability against the base H2O2 wet-etchant. Additionally, the base H2O2 wet-etchant results in an increase of the intensity ratio of the M–OH bond and a decrease of the M–O bond in the surface region of IZO films. In particular, these M–OH bonds are dominantly generated on the surface of the HDL 1 h + 1 h film, where the intensity ratio of the newly generated M–OH bond contributes to about 12.7% and 5.4% from the HDL 1 h + 1 h and HDL 3 h + 3 h, respectively, as shown in Fig. 4b, ESI Fig. S6 and S7.†
According to the Pourbaix diagram of Zn metal, the generated Zn2+ ions produce Zn(OH)2 products in an aqueous solution with alkaline environments, as follows:38
| Zn2+ + 2OH− → Zn(OH)2(aq.) | (4) |
Thus, we can expect that the weakly bonded Zn–O is preferentially broken in the base H2O2 wet-etchant, which abundantly generates Zn2+ ions and dangling bonds. Several Zn2+ ions will produce the Zn(OH)2 complex by combining with various OH− ions in the base H2O2 wet-etchant, as shown in Fig. 4c, and this etching process proceeds by penetrating along the pore sites in the IZO films (SDL 1 h and HDL 1 h + 1 h) with relatively weak M–O bonds.
Solution-processed a-IZO channel BCE TFT with a Mo S/D electrode
Generally, single spin-coated and short time-annealed metal oxide semiconductor channel TFTs that are fabricated using sol–gel methods have shown superior mobility and stability, despite a short thermal condensation time (around 1 h).2 To date, most studies have used unpatterned channels and an S/D electrode definition by a lift-off process and shadow mask. The solution-processed a-IZO TFTs did not experience soaking in the acidic or basic solutions. For the application of solution-processed a-IZO TFTs on large-area and mass production, the wet-etched pattern of the IZO channel and Mo electrode is necessary. However, if we do not use the etch-stopper layer for the solution processed a-IZO TFTs, the effect of the chemical etchant on the back surface of the IZO films should be considered based on the previous etching behaviors.Fig. 5 shows the transfer curves of solution-processed a-IZO TFTs (SDL 1 h, HDL 1 h + 1 h, and HDL 3 h + 3 h) with respect to the stacking process and annealing time. The Mo S/D electrodes are defined on the patterned IZO channels via a conventional wet-etch process and lift-off (see Fig. S8 in the ESI†), respectively, for comparison. Here, in the case of the SDL 1 h and HDL 1 h + 1 h samples, the a-IZO TFTs with Mo S/D electrodes defined using the wet-etch process with back-channel damage distinctly shows severe electrical degradation, compared to that using a conventional lift-off process without the back-channel damage. The lift-off process based a-IZO TFTs shows a tolerable mobility (0.6 < μFE < 2.6 cm2 V−1 s−1), threshold voltage (−10 < VTh < 0 V), and on–off ratios (108 < Ion/off < 109). In particular, the well-patterned a-IZO TFT (HDL 3 h + 3 h) with a high M–O ratio (61%), low porosity (22.7%), and smooth surface (2.74 Å) exhibits the best mobility and a remarkable uniformity (2.2–2.6 cm2 V−1 s−1). However, the SDL 1 h and HDL 1 h + 1 h TFTs with wet-etched Mo S/D electrodes lost their intrinsic transfer properties by applying the BCE process to define Mo electrodes with base H2O2 wet-etchant (pH 9.5). Since the degraded TFT performance is involved with the chemical back-channel damage, it indicates that the typical IZO preparations, such as thermal annealing at 400 °C for 1 h, is inadequate for the BCE processed a-IZO TFTs with channel pattern and chemically etched Mo electrodes.
Surprisingly, the HDL 3 h + 3 h TFT with wet-etched Mo S/D electrodes maintains its original transfer property, where the field-effect mobility is around 1.2–1.6 cm2 V−1 s−1, similar to that prepared with the lift-off Mo S/D electrode (2.2–2.6 cm2 V−1 s−1), as shown in Fig. 5. Considering the previous electrochemical corrosion results, the base H2O2 wet-etchant (pH 9.5) preferentially ionizes the Zn–O bond and induces a poor Zn compositional distribution. Unlike other samples, the Zn composition in the HDL 3 h + 3 h film with a strong M–O bond is nearly equivalent in the bulk region (ΔZn = 0%) or slightly reduced (ΔZn = 9%) in the surface region, compared to those in the as-dep film, implying an enhanced chemical durability (Fig. 4a). Among our samples, only the HDL 3 h + 3 h film showed superior chemical durability with minimum wet-etchant damage, but its μFE was slightly reduced, regardless of similar VTh and SS values. Based on the chemical composition analysis, only the surface region was slightly affected by the wet-chemical process, and it is responsible for the slight reduction of μFE. Thus, we tried to recover the electrical property of the HDL 3 h + 3 h TFT via post-annealing under N2 ambient conditions (400 Torr, 300 °C, and 10 min) for the surface modification (Fig. 6). Post-annealing under N2 ambient conditions can efficiently evaporate –OH radicals (Zn(OH)2 complex and –OH from alkaline H2O2) and re-align dangling bonds from the broken M–O bonds on the surface. As shown in the transfer curves of the HDL 3 h + 3 h TFT with the wet-etched Mo electrode (Fig. 6a), N2 post-annealing can almost recover the field-effect mobility (2.5 cm2 V−1 s−1) and on-current without the oxidation of the Mo electrode (Fig. 6b), up to the mobility level of the lift-off processed HDL 3 h + 3 h TFT. Furthermore, N2 post-annealing at 300 °C for 10 min induces no change to the ohmic contact or inter-diffusion at the electrode/channel, contrary to the conventional Al and Cu electrodes.39,40 Conclusively, the definition of popular Mo electrodes via a wet-etchant BCE process on the solution-processed IZO channels can be realized with superior chemical durability of the back channel in the base H2O2 wet-etchant (pH 9.5). However, we determined that these solution-processed a-IZO TFTs need the formation of more robust channels using multi-stacking IZO channel coatings and by performing sufficient thermal annealing for a prolonged time.
 | ||
| Fig. 6 Solution-processed indium zinc oxide (HDL 3 h + 3 h) thin film transistors with lift-off, wet-etched, and wet-etched/annealed Mo electrodes: (a) transfer and (b) output curves. | ||
Conclusions
In summary, we designed solution-processed IZO TFTs with chemically wet-etched Mo electrodes and high chemical durability patterned channels, in contrast to the TFTs using typical unpatterned sol–gel prepared channels and lift-off or shadow mask electrodes. In particular, we found that the IZO channels were vulnerable to wet chemical treatment and required a more delicate process design (pH, annealing time, channel film density, recovery process) to secure transfer behavior comparable to the typical TFT performances. The appropriate pH value for the incorporation of KOH in the H2O2 based wet-etchants was determined to be 9.5, because of low chemical damage to the IZO films, a fast Mo etch rate, and no Mo-related residues. To ensure the chemical stability from base H2O2 wet-etchants, the first spin-coated IZO bottom-layer was prepared by a soft-bake at 200 °C and a hard-bake at 400 °C for 3 h, and sequentially the second IZO top-layer was spin-coated with the same procedure. Consequently, despite Mo S/D electrodes defined via a wet-based BCE process and wet-chemical channel pattern, the solution-processed IZO (HDL 3 h + 3 h) TFT exhibited a slightly reduced field-effect mobility (1.2–1.6 cm2 V−1 s−1), compared to that prepared with lift-off process (2.2–2.6 cm2 V−1 s−1), due to a good chemical durability of the IZO back surface. Finally, additional appropriate N2 post-annealing recovered the original field-effect mobility (2.5 cm2 V−1 s−1) and on-current without the oxidation of Mo metals.Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grant funded by Basic Research Laboratory project of the Korea government (MSIP) (Grant No. 2014R1A4A1008474) and the Human Resources Program in Energy Technology of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (Grant No. 20154030200870).Notes and references
- K. K. Banger, Y. Yamashita, K. Mori, R. L. Peterson, T. Leedham, J. Rickard and H. Sirringhaus, Nat. Mater., 2011, 10, 45–50 CrossRef CAS PubMed.
- Y. H. Kang, S. H. Jeong, J. M. Ko, J. Y. Lee, Y. M. Choi, C. J. Lee and S. Y. Cho, J. Mater. Chem., 2014, 2, 4247–4256 CAS.
- C. Y. Lee, C. L. Chang, W. P. Shih and C. L. Dai, Thin Solid Films, 2009, 518, 3992–3998 CrossRef.
- H. Xu, L. F. Lan, M. Xu, J. H. Zou, L. Wang, D. Wang and J. B. Peng, Appl. Phys. Lett., 2011, 99, 2535501 Search PubMed.
- M. K. Kim, J. H. Jeong, H. J. Lee, T. K. Ahn, H. S. Shin, J. S. Park, J. K. Jeong, Y. G. Mo and H. D. Kim, Appl. Phys. Lett., 2007, 90, 212114 CrossRef.
- J. Y. Kwon, K. S. Son, J. S. Jung, K. H. Lee, J. S. Park, T. S. Kim, K. H. Ji, R. Choi, J. K. Jeong, B. W. Koo and S. Y. Lee, Electrochem. Solid-State Lett., 2012, 13, H213–H215 CrossRef.
- S. H. Ryu, Y. C. Park, M. Mativenga, D. H. Kang and J. Jang, Electrochem. Solid-State Lett., 2012, 1, Q17–Q19 CrossRef CAS.
- S. Tomai, M. Nishimura, M. Itose, M. Matuura, M. Kasami, S. Matsuzaki, H. Kawashima, F. Utsuno and K. Yano, J. Appl. Phys., 2012, 51, 03CB01 CrossRef.
- B. H. Seo, S. H. Lee, I. S. Park, J. H. Seo, H. H. Choe, J. H. Jeon, M. P. Hong, Y. U. Lee and J. Winkler, Thin Solid Films, 2011, 519, 6806–6809 CrossRef CAS.
- M. J. Zhao, L. F. Lan, H. Xu, M. Xu, M. Li, D. X. Luo, L. Wang, S. S. Wen and J. B. Peng, Electrochem. Solid-State Lett., 2012, 1, P82–P84 CrossRef CAS.
- K. Ide, Y. Kikuchi, K. Nomura, M. Kimura, T. Kamiya and H. Hosono, Appl. Phys. Lett., 2011, 99, 093507 CrossRef.
- W. H. Jeong, J. H. Bae and H. J. Kim, IEEE Electron Device Lett., 2012, 33, 68–70 CrossRef CAS.
- Y. H. Hwang, J. S. Seo, Y. M. Yun, H. J. Park, S. H. Yang, S. H. K. Park and B. S. Bae, NPG Asia Mater., 2013, 5, 1–8 Search PubMed.
- Y. H. Kim, J. S. Heo, T. H. Kim, S. J. Park, M. H. Yoon, J. W. Kim, M. S. Oh, G. R. Yi, Y. Y. Noh and S. K. Park, Nat. Mater., 2012, 489, 128–133 CrossRef CAS PubMed.
- M. G. Kim, M. G. Kanatzidis, A. Facchetti and T. J. Marks, Nat. Mater., 2011, 10, 382–388 CrossRef CAS PubMed.
- C. H. Kim, Y. S. Rim and H. J. Kim, ACS Appl. Mater. Interfaces, 2013, 5, 6108–6112 CAS.
- K. W. Jo and W. J. Cho, Phys. Status Solidi A, 2014, 12, 2817–2822 CrossRef.
- S. Y. Lee, T. Kang, S. M. Han and Y. S. Lee, Trans. Electr. Electron. Mater., 2015, 16, 46–48 CrossRef.
- Y. N. Gao, J. G. Lu, J. H. Zhang and X. F. Li, RSC Adv., 2015, 5, 37635–37639 RSC.
- J. M. Kwon, J. H. Jung, Y. S. Rim, D. L. Kim and H. J. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 3371–3377 CAS.
- S. J. Sung, S. J. Park, S. B. Cha, W. J. Lee, C. H. Kim and M. H. Yoon, RSC Adv., 2015, 5, 38125–38129 RSC.
- J. S. Seo and B. S. Bae, ACS Appl. Mater. Interfaces, 2014, 6, 15335–15343 CAS.
- W. Hu and R. L. Peterson, Appl. Phys. Lett., 2014, 104, 192105 CrossRef.
- K. T. Kim, J. K. Kim, Y. H. Kim and S. K. Park, IEEE Electron Device Lett., 2014, 35, 850–852 CrossRef CAS.
- W. T. Park, I. Y. Son, H. W. Park, K. B. Chung, Y. Xu, T. W. Lee and Y. Y. Noh, ACS Appl. Mater. Interfaces, 2015, 7, 13289–13294 CAS.
- K. M. Kim, C. W. Kim, J. S. Heo, H. I. Na, J. E. Lee, C. B. Park, J. U. Bae, C. D. Kim, M. C. Jun, Y. K. Hwang, S. T. Meyers, A. Grenville and D. A. Keszler, Appl. Phys. Lett., 2011, 99, 242109 CrossRef.
- Y. S. Rim, W. H. Jeong, D. L. Kim, H. S. Lim, K. M. Kim and H. J. Kim, J. Mater. Chem., 2012, 22, 12491–12497 RSC.
- S. H. Lee, B. H. Seo and J. H. Seo, J. Korean Phys. Soc., 2008, 53, 2603–2606 CrossRef CAS.
- A. G. Tyurin, Prot. Met., 2003, 39, 410–416 Search PubMed.
- D. X. Luo, H. Xu, M. Li, H. Tao, L. Wang, J. B. Peng and M. Xu, IEEE Electron Device Lett., 2014, 61, 92–97 CrossRef CAS.
- Y. H. Chung and C. W. Lee, J. Electrochem. Sci. Technol., 2012, 3, 1–13 CrossRef CAS.
- B. Beverskog and I. Puigdomenech, Corros. Sci., 1997, 39, 107–114 CrossRef CAS.
- J. W. Hennek, J. Smith, A. M. Yan, M. G. Kim, W. Zhao, V. P. Dravid, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2013, 135, 10729–10741 CrossRef CAS PubMed.
- M. G. Yun, C. H. Ahn, S. W. Cho, S. H. Kim, Y. K. Kim and H. K. Cho, ACS Appl. Mater. Interfaces, 2015, 7, 6118–6124 CAS.
- J. Socratous, K. K. Banger, Y. Vaynzof, A. Sadhanala, A. D. Brown, A. Sepe, U. Steiner and H. Sirringhaus, Adv. Funct. Mater., 2015, 25, 1873–1885 CrossRef CAS PubMed.
- M. Valtiner, S. Borodin and G. Grundmeier, Phys. Chem. Chem. Phys., 2007, 9, 2406–2412 RSC.
- A. Kelchtermans, K. Elen, K. Schellens, B. Conings, H. Damm, H.-G. Boyen, J. D'Haen, P. Adriaensens, A. Hardy and M. K. V. Bael, RSC Adv., 2013, 3, 15254–15262 RSC.
- D. D. Justice and R. M. Hurd, J. Electrochem. Soc., 1971, 118, 1417–1420 CrossRef CAS.
- Y. Xu, C. Liu, P. S. K. Amegadze, W. T. Park, D. X. Long, T. Minari, F. Balestra, G. Ghibaudo and Y. Y. Noh, Appl. Phys. Lett., 2014, 105, 133505 CrossRef.
- J. R. Yim, S. Y. Jung, H. W. Yeon, J. Y. Kwon, Y. J. Lee, J. H. Lee and Y. C. Joo, J. Appl. Phys., 2012, 51, 011401 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis procedure, transfer curves, surface optical microscopy images, electrochemical dissolution monitoring, angle resolved and X-ray photoelectron spectra, Fig. S1–S8. See DOI: 10.1039/c6ra09684k |
| ‡ Da Eun Kim and Sung Woon Cho are contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |